Marine Stainless Steel Applications
Abstract
Marine applications are very demanding. Stainless steel is the ideal materials for marine applications due to the fact that resists rust better than other materials such as brass, bronze, or galvanized steel. By now it is common knowledge that stainless steels ranging from AISI 316 up to 6Mo and superduplex do not always resist seawater.
This article describes the performance of stainless steel in marine environment, and how the performance can be improved.
Introduction
Marine applications are very demanding. Stainless steel is the ideal material for marine applications due to the fact that it resists rust better than other materials such as brass, bronze, or galvanized steel. By now it is common knowledge that stainless steels ranging from AISI 316 up to 6Mo and superduplex do not always resist seawater.
Crevice corrosion and pitting may develop sooner or later. For example, a 25Cr07Ni super duplex tubular heat exchanger in a marine vessel showed crevice corrosion within 6 months of service. In natural seawater a biofilm will develop on the metal surface and it will always promote the corrosivity of the water. Microbiological Induced Corrosion (MIC) often occurs in seawater. Also galvanic corrosion is a major problem at sea.
This article describes the performance of stainless steel in marine environment, and how the performance can be improved. Materials selection in marine environment is quickly gaining interest because of the worldwide trend to concentrate major industrial facilities around sea ports in order to save transport cost and increase cooling capacity.

Figure 1: Marine environment of stainless steels
Localised Corrosion
In marine environment stainless steel will never corrode uniformly. Corrosion is localized, i.e.: pitting corrosion and crevice corrosion. Localized corrosion often is being promoted by a biofilm. Figure 2 shows an example of crevice corrosion in a tubular heat exchanger.
Crevice corrosion is a major problem in marine environment because of the low resistivity of the water (seawater resistivity is about 0.35 Ohm•m). Even 6% Mo SS at 30°C can suffer crevice corrosion in seawater. If chlorinated, seawater below 25°C will not cause pitting corrosion to duplex stainless steel 2205 and alloys with higher PREN-value. PREN value is defined as ‘Pitting Resistance Equivalent Number’:
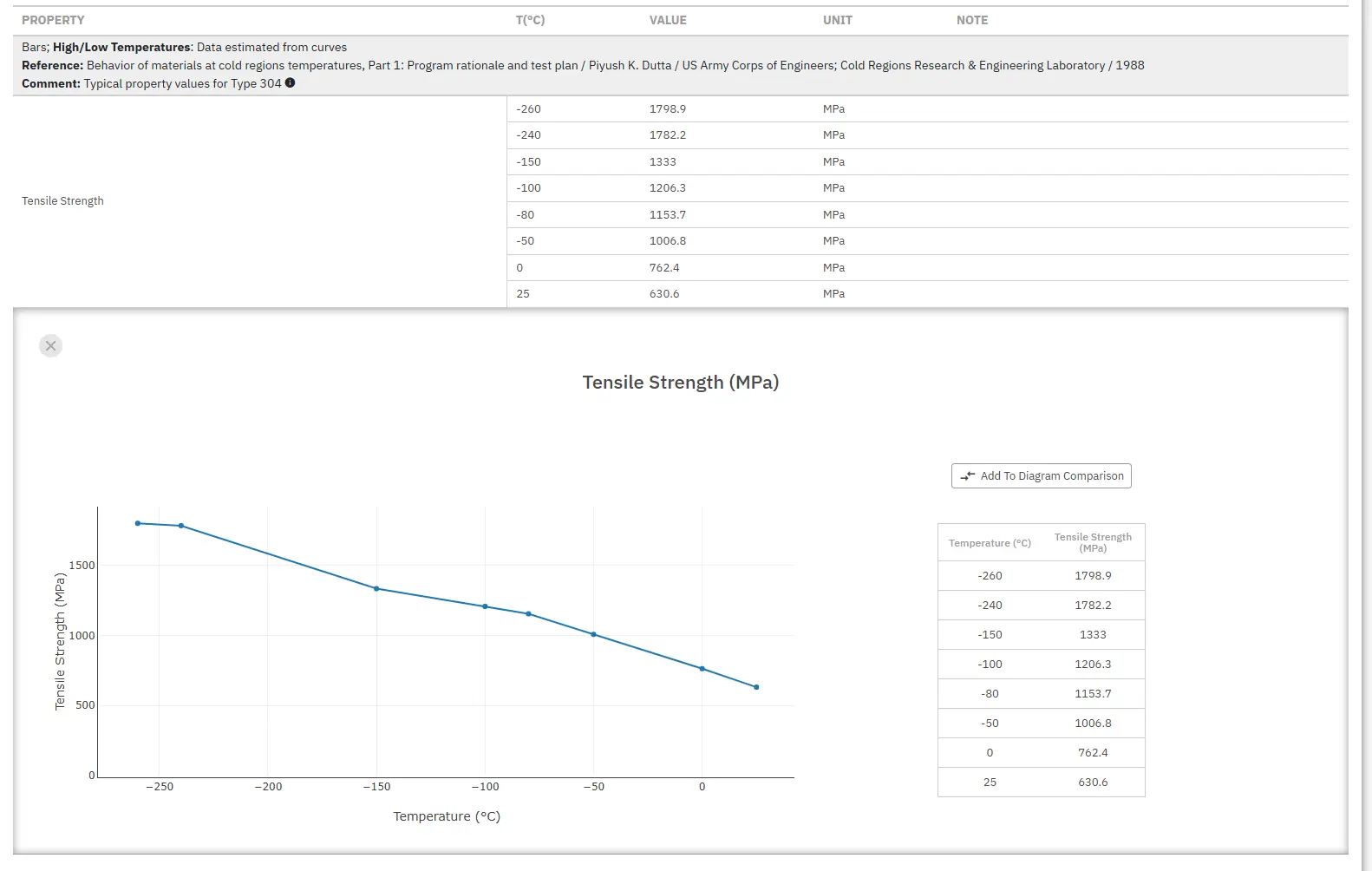
where X = 16 for duplex and X = 30 for austenitic steels. The higher the PREN value the better the pitting resistance.
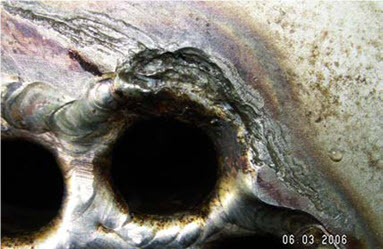
Figure 2: Crevice corrosion in seawater cooler. This corrosion finally leads to leakages.
Stress corrosion cracking
At temperatures above 60°C stainless steel 304 and 316 are sensitive to chloride cracking. Oxygen must be present, which means that produced water from oil or gas production does not cause stress corrosion cracking, even at high temperature. Duplex stainless steel and 6% Mo are much less sensitive to this phenomena, however under extreme conditions, i.e. high temperature and high stresses and cold deformation it may occur. Sometimes stress corrosion cracking occurs from the outside; especially longitudinal welded pipes at higher temperature are sensitive to this type of ‘corrosion under insulation’.
Galvanic Corrosion
Like crevice corrosion, the low resistivity of seawater also promotes strongly galvanic corrosion. Galvanic corrosion is seen as a major concern for materials performance in marine environment. A well known example is bronze bearings in ships, where sacrificial zinc anodes need to protect the steel hull for galvanic corrosion. Also stainless steel can suffer galvanic corrosion, or it causes galvanic corrosion to other, less noble, alloys.
Cathodic Protection of Stainless Steels
Most corrosion problems with stainless steel in marine environments can be avoided by use of cathodic protection. Both ‘supressed current’ and sacrificial anodes can achieve. The latter mentioned method normally is beneficial because it is more economical and less sensitive to failure. The current required for protecting the stainless steel only is a couple of mA/m2, compared to ~25 mA/m2 for carbon steel.
Carbon steel needs to be protected against uniform corrosion and therefore needs this high current, where stainless steel only needs to be polarized some 100 mV to keep the potential below the pitting potential in the safe ‘passive region’. If sacrificial anodes were connected to stainless steel, the current would be too high resulting in fast anode consumption and hydrogen charging of the stainless steel. The latter may lead to hydrogen embrittlement of duplex stainless steel, titanium (hydriding) and ferritic/martensitic stainless steels. For that reason, the anode current needs to be controlled by a resistor (RCP anodes) or a diode. Proper cathodic protection design is required to assure best performance.
External atmospheric corrosion
If uncoated, 316L piping and especially tubing suffer from rust formation and pitting, leading to leakages. Also pitting corrosion and chloride cracking, at temperatures above 60°C, occur under insulation material, provided it gets wet. For that reason, coating of the outside is recommended for piping. Materials selection for tubing normally is 6% Mo SS, which is resistant to corrosion from the outside. For the rest all uncoated 316 materials, such as instrument housing, will start to rust.
The Nisshin Steel Co, Ltd steel grade NSS445M2 is a ferritic stainless steel having superior antirust and weld anticorrosion characteristics which has been developed as a material suited for roofing and facing applications and for applications where high resistance to hot water containing chloride is required. The main constituent of this steel is 22Cr-1.2Mo. By adding Nb, Ti, and Al, the surface film of this steel is reinforced to improve its rust resistance. Further, the loss of Cr through oxidation at the time of welding is suppressed to prevent the weld from degrading in corrosion resistance. In addition to these features, its thermal expansion coefficient is nearly equal to that of common steel and smaller than that of austenitic stainless steel, so this steel is also suitable for long-size roofing applications.
Figure 3 shows the comparing results of atmospheric exposure test 445M2 grade to the common grades of austenitic stainless steel, 304 and 316 and to the feritic grade 444. The atmospheric exposure test was carried out in various Japanese marine sub-tropical areas. As can be seen from the Figure 3, the steel 445M2 was completely free of rusting after 2 years exposure even in highly corrosive environments such as Miyakojima.
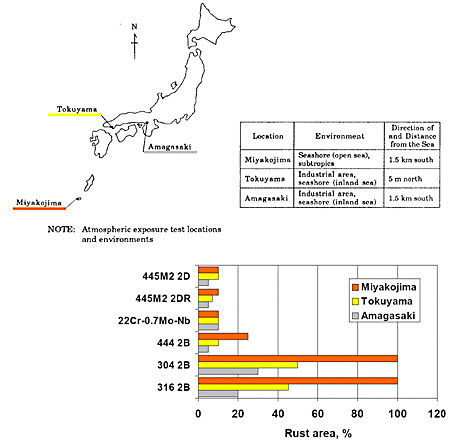


Figure 3: The comparing atmospheric exposure test results.
Uzyskaj dostęp do precyzyjnych właściwości stali nierdzewnych już teraz!
Total Materia Horizon zawiera informacje o właściwościach dla ponad 120 000 stali nierdzewnych: skład, właściwości mechaniczne i fizyczne, właściwości nieliniowe i wiele więcej.
Uzyskaj BEZPŁATNE konto testowe w Total Materia Horizon i dołącz do społeczności ponad 500 000 użytkowników z ponad 120 krajów.