Welding of Nickel-Base Corrosion-Resistant Alloys
Abstract
The nickel-chromium-molybdenum alloys (Hastelloy alloy C) proved to be extremely important in the chemical processing industry, because they provided corrosion resistance over a wide range of reducing and oxidizing environments.
Most commonly used process for joining corrosion-resistant (CR) alloys, and effects on alloys during welding process CR alloys: effect of weld metal segregation on corrosion resistance, propensity to form porosity, solidification hot-crack sensitivity.
The nickel-chromium-molybdenum alloys (Hastelloy alloy C) proved to be extremely important in the chemical processing industry, because they provided corrosion resistance over a wide range of reducing and oxidizing environments. However, grain precipitation of carbides in the as-welded condition required a postweld solution heat treatment to restore optimum corrosion resistance.
An important factor in this new alloy was the development of a reliable quantitative method for determining susceptibility to intergranular attack in the original C-type alloy and the newer C-276 and C-4 alloys. The maximum carbon content of alloy C-276 is 0,01% C with typical production compositions of about 0,005% C. Unfortunately, the optimum corrosion resistance of alloy C-276 was founded to be hampered by the precipitation of an intermetallic compound rich in molybdenum and tungsten.
Hastelloy alloy B-2 was originally developed to resist hydrochloric acid in all concentrations up to the boiling point and is used in many applications involving the production of this acid, as well as acetic acid and other chemicals. The alloy also resists sulfuric acid and pure phosphoric acid. Without chromium, however, this alloy is vulnerable to corrosion attack in reducing acids when oxidizing salts such as ferric or cupric chloride are present, even in the parts-per-million range.
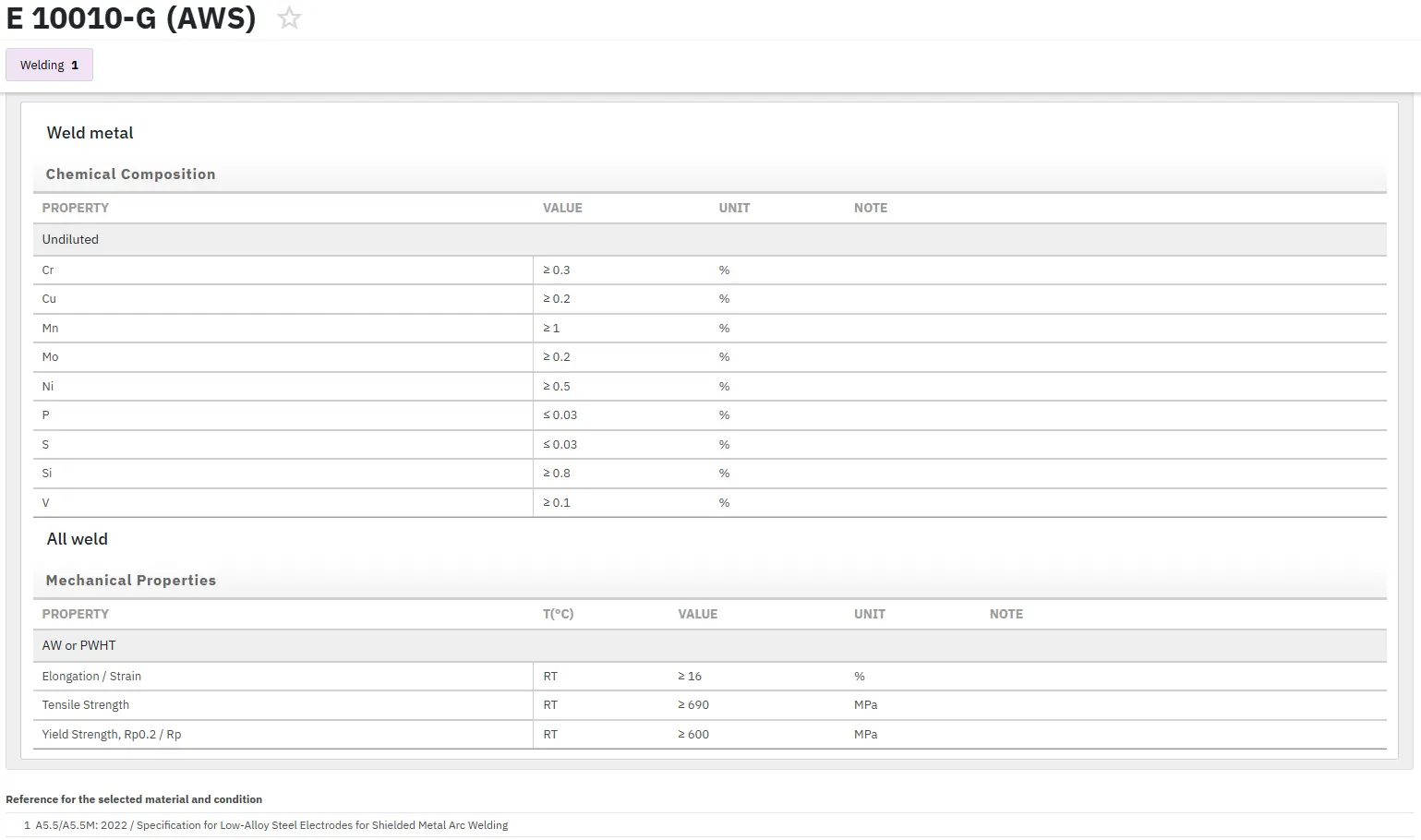
The G family of alloys has excellent resistance to phosphoric acid and has been used in wet-process phosphoric acid evaporators, agitator shafts, pumps, and in the handling of superphosphoric acid.
Because of similarities in heat capacity, density, and other physical properties, the welding characteristics of the alloys described in this article are quite similar to austenitic stainless steels. The major differences between these alloys and stainless steel are lower thermal expansion, lower thermal conductivity, and higher electrical resistivity. Generally, solidus temperatures are about 55oC lower than type 304 stainless.
Welding Processes
Gas-tungsten arc welding (GTAW), gas-metal arc welding (GMAW), and shielded-metal arc welding (SMAW) processes are commonly used to join this family of corrosion resistant (CR) alloys.
A filler metal that matches the composition of the base material is usually recommended when welding the nickel-chromium-molybdenum CR alloys. Other processes, such as plasma arc welding (PAW), resistance spot welding (RSW), laser-beam welding (LBW), electronbeam welding (EBW), and friction welding, can be used. The plasma arc cutting process is commonly used to cut alloy plate into desired shapes and to prepare weld angles. Oxyacetylene cutting is not recommended, nor is the use of oxyacetylene welding (OAW) and submerged arc welding (SAW). Oxyacetylene welding is not recommended because of the possibility of carbon pickup from the flame, whereas submerged arc welding is not recommended because of its high heat input, chromium loss across the arc, and silicon pickup from the welding flux.
Generally, welding heat input is controlled in the low-to-moderate range. Wide weave beads are not recommended. Stringer bead welding techniques, with some electrode manipulation, are preferred. The nickel-chromium-molybdenum alloy weld metal is not as fluid as carbon steel and does not flow out as readily to "wet" the sidewalls.
Therefore, the welding arc and filler metal must be manipulated so as to place the molten metal where it is needed. In addition to sluggishness, the penetration pattern of this alloy type is less than that of a typical carbon or stainless steel weld, and incomplete fusion is more likely to occur. Therefore, care must be taken to ensure that the groove opening is wide enough to allow proper torch or electrode manipulation and proper placement of the weld bead.
Welding Metallurgy
The net effect is that during the welding of low carbon nickel-molybdenum and nickel-chromium-molybdenum CR alloys, Heat-Affected Zone (HAZ) grain boundary precipitation is still a potential reality, despite the relatively low carbon content of these alloys. The amount and severity of precipitation will depend on the cooling rate through the intermediate temperature range from 1000 to 600oC.
Fusion zone welding metallurgy of the nickel-molybdenum and nickel-chromium-molybdenum alloy family is important, because base materials are usually welded with filler metals of matching composition and because these alloys can be welded autogeneously (no filler added), as in the case of welded tubular products.
Three issues should be considered in terms of the fusion zone:
- Effect of weld metal segregation on corrosion resistance
- Propensity to form porosity
- Solidification hot-crack sensitivity.
Segregation. Because of the segregation of solute elements upon solidification (principally molybdenum, which segregates to the cellular dendritic boundaries of the fusion zone), it is generally accepted that the corrosion resistance of the weld metal will be marginally less than that of the more homogeneous wrought base material. Differences in corrosion performance, however, depend heavily on the severity of the corrosion environment.
Porosity. Recognizing that the solubility of gas is greater in the liquid state than in the solid state, the subject of weld porosity is relevant to fusion zone discussion. The three most logical origins of gas-generated porosity are carbon monoxide, hydrogen, and nitrogen. However, in welding the low-carbon nickel-molybdenum and nickel-chromium-molybdenum alloys, the cause of porosity is not believed to be from the formation of carbon monoxide. As long as there is a residual silicon or aluminum content in the weld deposit, the oxygen potential is too low for the formation of carbon monoxide.
Gas hole formation can be caused by hydrogen evolution. As is well known, even the smallest amount of water will be reduced by metal deoxidizers, releasing a sufficient volume of gas to create porosity. Hydrocarbons can also dissociate and go into solution.
Znajdź natychmiast tysiące materiałów spawalniczych!
Total Materia Horizon zawiera tysiące materiałów nadających się do spawania i elektrod, z ich właściwościami w stanie luzem i w stanie spawanym.
Uzyskaj BEZPŁATNE konto testowe w Total Materia Horizon i dołącz do społeczności ponad 500 000 użytkowników z ponad 120 krajów.