Effect of small additions of Ag, Bi, Cu and Sb on structure and Solder properties of Sn-Zn eutectic alloy: Part One
by M.Kama and E.S.Gouda
Abstract
One of the key issues in the electronics packaging industries is the serious environmental problems associated with the lead-containing solders.
The goal in the development of lead-free solder is to produce alloys with nearly the same properties as those of the lead-containing solder alloys.
Abstract
The effects of third elements, i.e., Ag, Bi, Cu and Sb on structure and the mechanical properties of the Sn–9Zn lead- free solder alloy were investigated. The results indicate that Ag and Cu combine with Sn and Zn to form Ag-Sn, Ag-Zn, Cu-Sn and Cu-Zn compounds, respectively. These compounds act as hard inclusions in the matrix then increase the hardness of the two alloys. Also, these compounds may act as scattering centers for conduction electrons, hence increase the electrical resistivity of these two alloys. Whatever, additions of Bi and Sb, led to complete solubility of the two metals in the Sn-matrix? This complete solubility may cause local internal strains in the Sn- matrix. These strains tend to decrease the hardness of the two alloys. The change of dissolving atoms in the Sn-matrix as substitutional solid solutions, which have different mobility due to change in their atomic radii, in addition to the change of the axial ratio (c/a), of Sn-matrix change the internal friction of these alloys.
Introduction
One of the key issues in the electronics packaging industries is the serious environmental problems associated with lead-containing solders. This has resulted in extensive research work to develop lead-free solders with suitable soldering characteristics and mechanical properties. The goal in the development of lead-free solder is to produce alloys with nearly the same properties as those of the lead-containing solder alloys. There are some characteristics which play a major role in consideration of substituting tin-lead solders in electronic soldering: lower melting point, adequate strength, environmental issues related to the toxicity, good electrical/thermal conductivity, low cost, good wetting properties and availability in sufficient quantities depending on the base metals. There are many lead-free solder alloys containing Sn as the base metal with different alloying elements such as: Ag, Zn, Cu and Bi, which have been developed [1-5]. The Sn-Zn eutectic alloy has recently been considered as one of the most lead-free solder materials that can replace the Sn-37Pb solder without increasing soldering temperature. Sn-Zn alloy has greater mechanical properties than the conventional Sn-Pb solders [6-10]. The objective of this study aims to investigate the effect of small additions of Ag, Bi, Cu and Sb on structure and properties of Sn–9Zn eutectic lead-free solder alloy.
Experimental
Four of the alloying elements i.e., 1wt. % of Ag, Bi, Cu and Sb, were added separately to Sn-9Zn eutectic lead-free solder alloy. The alloys were prepared by melting pure tin and zinc as base metals with the metals (Bi, Cu, and Sb) of purities 99.99 % in a high frequency induction furnace. Then, the molten alloys were poured on a rotating wheel of the melt-spinning apparatus of linear speed 31.7 m/s. The resulting alloys had long ribbon shape of about 50 µm in thickness and about 1 cm in width. The structure of these alloys was examined by X-ray diffraction technique using a Shimadzu X-ray Diffractometer with Co-radiation. A dynamic resonance circuit was used to calculate the elastic modulus [11, 12] after determining the resonance frequency using the equation;
Where, d density of the sample under test, l, length of the vibrated part of the sample, (the distance from the contact point of the electromagnetic mechanical vibrator with the sample to its free end), t, thickness of the test sample. The hardness measurements were carried out using Vickers micro hardness tester. The electrical resistivity was calculated using the double bridge circuit.
Results and Discussion Structure
Figure 1 shows the X-ray diffraction patterns of the rapidly solidified Sn-9Zn-1X (X = 0, Ag, Bi, Cu, or Sb) lead-free solder alloys. Figure1a shows the peak positions of the Sn-9Zn eutectic alloy. It shows that, all X-ray diffraction peaks of Sn appeared. Also, there is a small X-ray diffraction peak due to Zn as secondary phase that appeared at 2θ = 50.62°. Adding 1wt.%Ag the pattern indicates double X-ray diffraction peaks due to the intermetallic compounds Ag3Sn and AgZn at 2θ = 44.8 and 85.3°, respectively in addition to the same phases. On the other hand, the alloy Sn-9Zn-1Bi has no any peak characteristic of Bi phase, which means complete solubility of Bi in the Sn matrix. While, adding 1wt. % Cu the pattern indicates a single X-ray diffraction peak due to the intermetallic compound γ-Cu5Zn8 at 2θ =75°. Moreover, the diffraction peak of Sn at 2θ =74.17° appeared as it is composed of two super imposed peaks, one due to Sn and the other due to the intermetallic compound η-Cu6Sn5. Adding Sb to the eutectic alloy lead to more precipitations of Zn as a secondary phase and there are no peak characteristic of the Sb phase, which means complete solubility of Sb in the Sn matrix. Table 1 shows the variation of the lattice parameters a, c, and c/a of the unit cell of -Sn in -Sn the Sn-9Zn-1X (X= 0, Ag, Bi, Cu, or Sb) alloys. It shows that, the lattice parameters a and c of -Sn are equal to 5.84 and 3.186 A, respectively with c/a = 0.5455. All additions decrease these values except in the case of Bi atoms. The contradiction in this case can be attributed to the larger of the atomic size of Bi than that of the Sn-matrix phase.
| Alloy | Lattice Parameter (α) A | Lattice Parameter (c) A | c/α | Volume of the unit cell A3 |
| Sn-9Zn | 5.840 | 3.186 | 0.5455 | 108.7 |
| Sn-9Zn-1Ag | 5.826 | 3.177 | 0.5453 | 107.8 |
| Sn-9Zn-1Bi | 5.843 | 3.189 | 0.5458 | 108.9 |
| Sn-9Zn-1Cu | 5.8422 | 3.179 | 0.5460 | 107.7 |
| Sn-9Zn-1Sb | 5.8426 | 3.178 | 0.5455 | 107.9 |
Elastic Modulus
Table 2 shows the values of the elastic modulus of the Sn-9Zn-1X (X = 0, Ag, Bi, Cu, or Sb) rapidly solidified alloys calculated from the resonance curves as in Figure 2, obtained by the dynamic resonance circuit after determination of the resonance frequency. It shows that, the value of Young’s modulus of the eutectic alloy is equal to 37.2 GPa. This value decreases to the minimum value of 27.1 GPa for the alloy Sn-9Zn-1Ag, while takes its maximum value 46.2 GPa for the alloy Sn-9Zn-1Cu. This behavior can be attributed to the change in the c/a ratio of the unit cell of β-Sn as illustrated in Figure 3. It shows that, Young’s modulus increases as the c/a ratio increases which confirmed elsewhere [13,14]. Increasing the c/a ratio means stretching the unit cell along the c-axis. This in turn may increase the bond strength. Moreover, the maximum value was obtained for the alloy Sn-9Zn-1Cu can be attributed to the formation of the intermetallic compound γ-Cu5Zn8 that has a higher modulus than that of either of the metallic components, in which the higher value can be attributed to the combination of ionic/covalent bonding in the intermetallic compound [15].
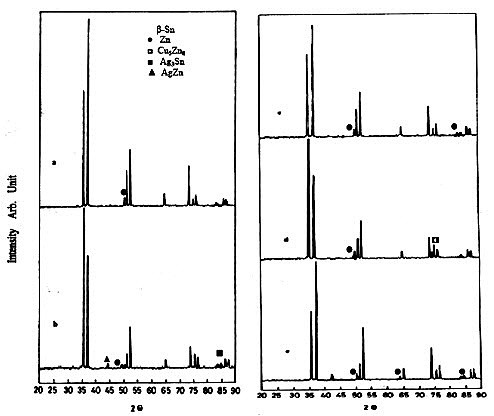
Figure 1: The X-ray diffraction patterns of the rapidly solidified Sn-9Zn-1X (X = 0, Ag, Bi, Cu, or Sb) lead-free solder alloys
| Alloy | Young's Modulus(E), GPa | Bulk Modulus(B), GPa | Shear Modulus(S), GPa | B/S |
| Sn-9Zn | 37.2±1.3 | 36.5 | 14.0 | 2.61 |
| Sn-9Zn-1Ag | 27.1±1.0 | 26.6 | 10.2 | 2.61 |
| Sn-9Zn-1Bi | 45.3±1.2 | 44.4 | 17.0 | 2.61 |
| Sn-9Zn-1Cu | 46.2±1.5 | 45.3 | 17.4 | 2.60 |
| Sn-9Zn-1Sb | 37.2±1.4 | 36.5 | 14.0 | 2.61 |
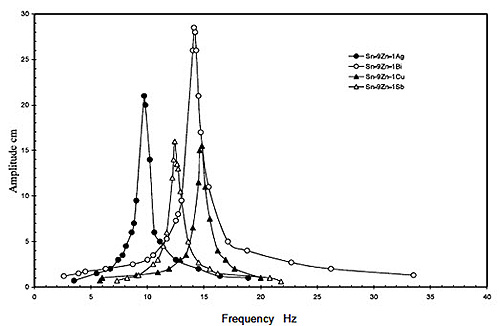
Figure 2: Resonance curves of the Sn-9Zn-1X (x = Ag, Bi, Cu or Sb) alloys
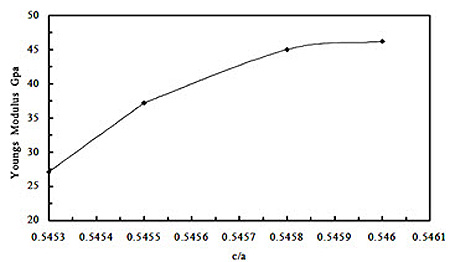
Figure 3: Variation of Young's modulus of the Sn-9Zn-1X (x= 0, Ag, Bi, Cu or Sb) alloys with c/a of the Sn-matrix
1 Metal Physics Lab., Psysics Department, Faculty of Science, Mansoura University, Egypt.
2 Department of Solid State Physiscs, Physiscs, Division, National Research Center, Dokki, Giza, Egypt.
3 Physiscs Department, Faculty of Science, Jazan University, Gizan, K.S.A.
Znajdź natychmiast tysiące materiałów spawalniczych!
Total Materia Horizon zawiera tysiące materiałów nadających się do spawania i elektrod, z ich właściwościami w stanie luzem i w stanie spawanym.
Uzyskaj BEZPŁATNE konto testowe w Total Materia Horizon i dołącz do społeczności ponad 500 000 użytkowników z ponad 120 krajów.