Effect of Phosphorus on the Properties of Carbon Steels: Part One
Abstract
Phosphorus plays a dual role in the properties of carbon steels, providing both beneficial and detrimental effects. While it enhances strength and certain manufacturing characteristics, excessive phosphorus can lead to embrittlement, reducing toughness and ductility. This article discusses the impact of phosphorus on various steel grades, its influence on mechanical properties, and the trade-offs involved in its use. A comprehensive overview of phosphorus levels is provided, detailing the requirements for different applications, including low-strength and high-strength steels.
Introduction
Phosphorus is a significant alloying element in steel, known for its ability to strengthen ferrite. The addition of merely 0.17% phosphorus can elevate both the yield and tensile strength of low-carbon sheet steel by approximately 62 MPa (9 ksi). This enhancement also improves bake hardening response and deep drawability, making rephosphorized high-strength steels popular in cold-forming applications. Additionally, phosphorus is utilized as an additive to enhance machining characteristics and atmospheric corrosion resistance.
Beneficial Effects of Phosphorus
Phosphorus is recognized as one of the most effective solid-solution strengtheners of ferrite. Its addition can lead to significant improvements in the mechanical properties of steel, as shown in Table 1.
| PROPERTY | EFFECT |
| Strength | Strong increase |
| Bake hardenabillity | Increase |
| Ductility | Strong decrease |
| Texture (R-value) | Depends on composition and processing |
| Coating behavior: | |
| Fe-Zn Galvanneal | Demands control of phosphorus. Can improve powdering. |
| Phosphatability | May improve |
| Enameling steels | Improves fishscaling. Accelerates pickling. |
| Spot weldability | Not harmful up to ~0.1% |
| Core loss of motor lamination steel | Strong decrease (improvement) |
| Embrittlement | Aggravates |
Table 1: Summary of the Effects of Phosphorus on Various Steel Grades
Detrimental Effects of Phosphorus
Despite its advantages, phosphorus can also lead to embrittlement, significantly reducing toughness and ductility. A well-known issue is temper embrittlement, which occurs in heat-treated low-alloy steels due to the segregation of phosphorus and other impurities at prior austenite grain boundaries. This phenomenon, along with the role of certain alloying elements, has been the focus of extensive research for several decades.
Intergranular Embrittlement
Phosphorus can cause two forms of intergranular embrittlement in steels with normal phosphorus levels (0.008% to 0.025%). In both cases, fractures occur along ferrite grain boundaries, weakened by phosphorus segregation during slow cooling or final annealing after cold rolling. One form results in planar-oriented cracking, while the other leads to brittle fracture during secondary cold working of previously deep-drawn sheet steel.
Hardenability Considerations
Research has shown that while the effects of phosphorus on hardenability vary, the standard methods for calculating hardenability from steel composition (ASTM A255 and SAE J406) do not typically account for phosphorus. This omission is largely because the effects of phosphorus and sulfur tend to balance each other out. Due to its negative impacts on the toughness and ductility of heat-treated steels, phosphorus is generally not added to enhance hardenability.
Phosphorus Levels in Steel
Very Low Phosphorus (Up to 0.02%)
Steels with very low phosphorus content are particularly susceptible to embrittlement, especially high-strength, low-alloy steels containing elements such as manganese, silicon, titanium, niobium, vanadium, molybdenum, and chromium. The trend is shifting towards reducing residual phosphorus levels, especially in line pipe steels, where a maximum phosphorus content of 0.02% is necessary, and 0.01% is desirable for corrosive gas transmission.
Moderately Low Phosphorus (0.02% to 0.03%)
Products that can tolerate 0.02% to 0.03% phosphorus include low-strength grades and high-strength steels not exposed to hostile environments. For commercial quality plain-carbon steels with minimal formability requirements, the upper limit can be somewhat relaxed. However, maintaining tight control over phosphorus levels is crucial for product consistency.
High Phosphorus (Rephosphorized)
Phosphorus is typically added as a strengthening agent in amounts up to 0.1%, with cold-rolled motor lamination steels sometimes containing up to 0.155% phosphorus to reduce AC core loss. The sensitivity of many properties to phosphorus content necessitates strict control over phosphorus levels and careful selection of processing methods to achieve consistent properties and avoid embrittlement.
Strength and Work Hardening
Phosphorus is a potent solid solution strengthener for ferrite. Small phosphorus additions can significantly enhance the strength of low-carbon sheet steel. For instance, an addition of 0.1 wt.% phosphorus can increase yield strength by approximately 62 MPa (9 ksi). Furthermore, phosphorus affects grain size in ferrite, particularly in ultra-low carbon steels.
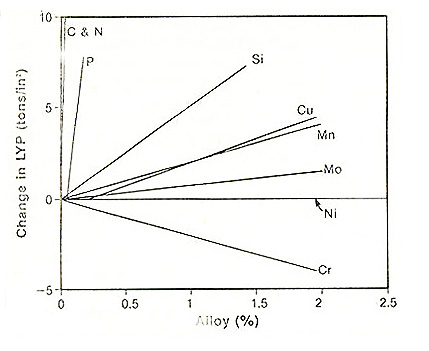
Figure 1: Change in the lower yield strength of carbon steel as a function of the alloy content for several common elements (1ton/inch2 = 13.8 MPa)
Cold working typically increases the strength of metals through work hardening. While phosphorus tends to reduce the work-hardening rate of ferrite, its impact is relatively minor at low concentrations.
Bake Hardening
Bake hardening occurs when certain steels undergo heating after plastic deformation, which can be economically utilized during the plant-bake cycle of formed sheet steel parts. Phosphorus enhances bake hardening, particularly in low-carbon, Al-killed steels. Grain refinement during annealing, attributed to phosphorus, significantly contributes to this effect.
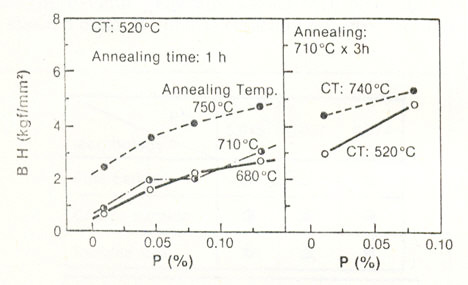
Figure 2: Effect of phosphorus content on the bake hardening increment in 0.04 Al-killed steels (1kg/mm2 = 9.8 MPa)
Ductility
While phosphorus strengthens steel, it simultaneously decreases ductility. The balance between strength and formability is crucial in designing high-strength formable steels. At elevated phosphorus levels, ductility can be severely limited due to embrittlement phenomena associated with phosphorus segregation at grain boundaries.
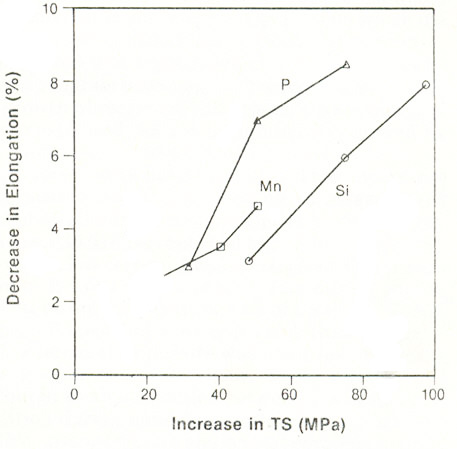
Figure 3: Decrease in ductility (percent total elongation) with tensile strength for P, Si and Mn-strengthened Ti-stabilized, ultra low carbon steels
Conclusion
In summary, phosphorus is a critical element in the composition of carbon steels, offering both advantages and challenges. Understanding its effects is essential for optimizing steel properties for various applications, ensuring a balance between strength, ductility, and formability. As the industry continues to evolve, ongoing research and development will be vital to address the challenges posed by phosphorus in steel manufacturing.
Znajdź natychmiast dokładne składy materiałów!
Total Materia Horizon zawiera składy chemiczne setek tysięcy materiałów i substancji, a także ich właściwości mechaniczne i fizyczne i wiele więcej.

Uzyskaj BEZPŁATNE konto testowe w Total Materia Horizon i dołącz do społeczności ponad 500 000 użytkowników z ponad 120 krajów.