The Effects of Alloying Elements on Iron-Carbon Alloys
Abstract
This article examines the fundamental effects of alloying elements on iron-carbon alloys, focusing on their influence on phase transformations and microstructural development. The article presents Wever's classification of iron binary equilibrium systems into four distinct categories: open γ-field, expanded γ-field, closed γ-field, and contracted γ-field systems. The analysis explores how various alloying elements affect the stability of austenite and ferrite phases, their distribution in steels, and their role in carbide formation. Understanding these relationships is crucial for controlling steel properties through heat treatment and composition manipulation, with significant implications for industrial applications and materials engineering.
Understanding the Classification of Iron-Carbon Alloy Systems
The systematic analysis of alloying elements' effects on iron-carbon alloys requires examining numerous ternary alloy diagrams across various temperature ranges. However, Wever's groundbreaking research established that iron binary equilibrium systems can be categorized into four fundamental groups, simplifying this complex subject. These categories are determined by how alloying elements influence the gamma (γ) phase field, commonly known as austenite.
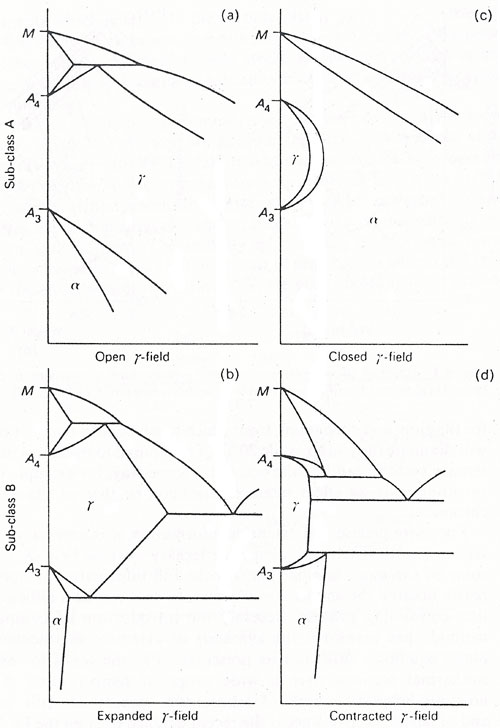
Figure 1: Classification of iron alloy phase diagrams: a. open γ-field; b. expanded γ-field; c. closed γ-field
Alloying elements primarily influence the equilibrium diagram in two distinct ways:
- As γ-stabilizers, expanding the γ-field and promoting austenite formation across broader compositional ranges
- As α-stabilizers, contracting the γ-field and encouraging ferrite formation over wider compositional limits
The classification's effectiveness is largely attributed to the electronic structure of the alloying elements, which correlates with their positions in the periodic table.
Effects of Different Classes of Alloying Elements
Class 1: Open γ-field Systems
The open γ-field category includes critical steel alloying elements such as nickel and manganese, alongside cobalt and noble metals (ruthenium, rhodium, palladium, osmium, iridium, and platinum). When added in sufficient quantities, nickel and manganese completely eliminate the body-centered cubic (bcc) α-iron phase, replacing it with the γ-phase down to room temperature (Fig1a). These elements lower both Ac1 and Ac3 transformation temperatures, facilitating the retention of metastable austenite during quenching. This property makes them particularly valuable in developing austenitic steels.
Class 2: Expanded γ-field Systems
Carbon and nitrogen stand as the most significant elements in this category. While they expand the γ-phase field, their range is limited by compound formation. Similar effects are observed with copper, zinc, and gold. The expansion of the γ-field by carbon and nitrogen is fundamental to steel heat treatment processes, enabling the formation of homogeneous solid solutions containing up to 2.0 wt% carbon or 2.8 wt% nitrogen in austenite (Fig.1b).
Class 3: Closed γ-field Systems
Several elements restrict γ-iron formation, creating a contracted area known as the gamma loop (Fig.1c). This category includes:
- Silicon, aluminium, beryllium, and phosphorus
- Strong carbide-forming elements: titanium, vanadium, molybdenum, and chromium
These elements promote bcc iron (ferrite) formation, resulting in continuous δ- and α-phase fields. Consequently, alloys containing these elements cannot undergo conventional heat treatments involving the γ/α-phase transformation.
Class 4: Contracted γ-field Systems
Boron leads this category, along with carbide-forming elements such as tantalum, niobium, and zirconium. The distinguishing feature is a strongly contracted γ-loop accompanied by compound formation.
Distribution and Behavior of Alloying Elements in Steel Systems
Ternary Systems and Element Interactions
While the previous analysis focused on binary systems, the addition of carbon creates more complex ternary systems. However, the general principles typically remain applicable. For any fixed carbon content, the addition of alloying elements either expands or contracts the γ-field based on the specific solute characteristics.
Consider these key interactions:
- Silicon addition restricts the γ-field while enlarging the α-field
- Vanadium contracts the γ-field and promotes vanadium carbide formation in equilibrium with ferrite
- Nickel, which doesn't form carbides, expands the γ-field
Interestingly, elements with opposing tendencies generally neutralize each other at appropriate combinations. However, exceptions exist, such as the synergistic effect of chromium with nickel in 18Cr8Ni austenitic steels, where chromium actually helps stabilize the γ-phase at around 18% concentration.
Quantitative Analysis of Phase Fields
A practical method for quantitatively illustrating alloying elements' effects involves projecting the γ-phase field boundaries onto the Fe-C plane of the ternary system. For more detailed analysis, researchers examine isothermal sections in true ternary Fe-C-X systems. However, complete data isn't always available due to the complexity and time-consuming nature of accurate data acquisition.
Recent advances in computer-based methods have revolutionized this field by enabling:
- Synthesis of extensive thermochemical data
- Analysis of phase equilibria information
- Generation of isothermal sections across wide temperature ranges
Classification of Elements in Transformable Steels
In transformable steels—where austenite converts to ferrite and carbide during slow cooling—the behavior and distribution of alloying elements follow distinct patterns. Understanding these patterns is crucial for controlling steel properties and predicting microstructural development.
Ferrite-Phase Elements
Elements such as nickel, copper, phosphorus, and silicon demonstrate a strong preference for the ferrite phase. These elements maintain minimal solubility in cementite or alloy carbides, instead remaining predominantly in solid solution within the ferrite phase. This characteristic makes them particularly effective for modifying ferrite properties without significantly affecting carbide formation.
Dual-Phase Elements
The majority of steel alloying elements, including manganese, chromium, molybdenum, vanadium, titanium, tungsten, and niobium, exhibit more complex behavior. These elements act as carbide formers and participate in multiple phases:
- At low concentrations, they dissolve into cementite and create solid solutions
- They maintain significant solubility in the ferrite phase
- At higher concentrations, they form thermodynamically stable alloy carbides
Manganese presents a unique case within this category. Rather than forming manganese carbide, it preferentially enters into solid solution in Fe3C. Most carbide-forming elements exist in quantities exceeding their carbide-forming requirements, with excess amounts entering ferrite solution alongside non-carbide forming elements like nickel and silicon.
Carbide-Phase Elements
The third category comprises elements that predominantly enter the carbide phase, with nitrogen being the most significant example. These elements form carbo-nitrides with iron and various alloying elements. In the presence of strong nitride-forming elements like titanium and aluminum, distinct alloy nitride phases may develop.
Microstructural Evolution and Phase Transformations in Alloyed Steels
Fundamental Transformation Patterns
The introduction of alloying elements into iron-carbon systems triggers significant microstructural modifications that profoundly influence steel properties. These transformations follow distinct patterns depending on the alloying elements' characteristics and their concentrations. Understanding these patterns is crucial for controlling steel properties and predicting material behavior.
Non-Carbide Forming Elements
Elements such as nickel, silicon, and manganese maintain the basic transformation patterns while modifying specific properties. These elements, which don't compete with cementite formation, preserve fundamental microstructural characteristics while enhancing particular attributes through solid solution strengthening. This behavior enables predictable heat treatment responses and controlled property modification.
[Note: A comparative microstructure image showing the effects of these elements would be valuable here]
Carbide-Forming Element Influences
Strong carbide-forming elements create more dramatic microstructural changes, following a clear hierarchy of influence. Moderate carbide formers like molybdenum, chromium, and tungsten gradually replace cementite with their respective alloy carbides at relatively low concentrations. More aggressive carbide formers—niobium, titanium, and vanadium—form alloy carbides below 0.1 wt%, fundamentally altering the steel's microstructure.
Solubility Considerations and Practical Implications
The relationship between carbon solubility in austenite versus ferrite significantly impacts microstructural development. While cementite shows high solubility in austenite compared to ferrite, most alloy carbides and nitrides demonstrate notably lower austenite solubility. This characteristic influences both heat treatment possibilities and final properties.
Practical solubility limits vary markedly among alloying elements:
- Niobium and titanium reach dissolution limits in austenite above 0.25 wt%
- Vanadium maintains effectiveness up to 1-2%
- Molybdenum remains soluble to approximately 5%
- Chromium exhibits higher solubility limits
These limits assume sufficient carbon availability; when carbon is limited, excess metallic elements distribute between austenite and ferrite phases.
Morphological Development
Transformation conditions significantly influence final morphologies. Slower transformation rates produce fibrous structures approaching equilibrium conditions, while rapid transformations, particularly in microalloyed steels, generate interphase precipitation and dislocation-nucleated structures. This relationship between transformation kinetics and resulting structures provides a powerful tool for property control.
Industrial Applications and Property Control
Understanding these structural changes enables:
- Precise heat treatment process design
- Accurate property prediction
- Optimized alloy composition development
- Controlled transformation product formation
This comprehensive knowledge of transformation behavior and structural evolution forms the foundation for modern steel design and processing, ensuring optimal material performance across diverse applications.
Transformation Characteristics and Microstructural Features in Alloyed Steels
Pearlitic Transformations and Nodular Formations
The most direct parallel to traditional pearlite formation occurs when alloy carbides develop a lath morphology, forming nodules in conjunction with ferrite. These pearlitic nodules typically emerge at temperatures just below Ac1, particularly in steels exhibiting relatively slow transformation rates. Chromium steels containing 4-12% chromium demonstrate this behavior distinctly, with crystallography analogous to cementitic pearlite, though with notable structural variations due to different carbide crystal structures.
Interphase Precipitation Mechanisms
Interphase precipitation represents a unique transformation mechanism characterized by periodic nucleation at the γ/α interface during transformation. This process produces distinctive features:
- Precipitate particles form in parallel bands following the interface direction
- Particle bands maintain alignment even through sharp directional changes
- Specific Widmanstätten variants often dominate in localized regions
The scale of these precipitate formations varies significantly among different alloy systems. Vanadium, titanium, and niobium steels typically exhibit extremely fine precipitate distributions due to rapid γ/α transformation rates. Higher transformation temperatures or the addition of elements like nickel and manganese that slow the reaction lead to coarser structures.
Scale Variations in Different Alloy Systems
The precipitate dispersion scale follows a consistent pattern across alloy systems:
- Coarsest in chromium, tungsten, and molybdenum steels (slower transformation)
- Intermediate in standard alloy steels
- Finest in vanadium, niobium, and titanium steels (rapid transformation)
Time-Temperature-Transformation (TTT) Characteristics
The transformation of austenite below the eutectoid temperature is best understood through isothermal transformation diagrams. These TTT curves provide essential quantitative information for heat treatment processes. In eutectoid plain carbon steel, the curve exhibits a characteristic C-shape, with pearlite formation occurring down to and slightly beyond the curve's nose. Lower temperatures promote bainite and martensite formation.
Complexities in Multi-Component Systems
For hypo- and hyper-eutectoid alloys, the transformation diagrams become more intricate, requiring additional curves to represent ferrite or cementite reactions. Understanding these relationships enables:
- Precise control of transformation products
- Optimization of heat treatment parameters
- Prediction of final microstructural features
- Enhanced property control in complex alloy systems
The practical application of this knowledge allows metallurgists to design heat treatment processes that achieve specific microstructural objectives while maintaining process efficiency and reproducibility.
Uzyskaj dostęp do dokładnych właściwości żeliw już teraz!
Total Materia Horizon zawiera informacje o właściwościach ponad 11 000 żeliw: skład, właściwości mechaniczne i fizyczne, właściwości nieliniowe i wiele więcej.
Uzyskaj BEZPŁATNE konto testowe w Total Materia Horizon i dołącz do społeczności ponad 500 000 użytkowników z ponad 120 krajów.