Electrowinning Process: Part One
Abstract
Electrowinning is an electrolytic process used to recover valuable metals from electroplating rinse waters while reducing wastewater generation and chemical discharge. This article explores the fundamental components of electrowinning systems, including electrolytic cells, rectifiers, and pumps, and explains their operational principles. The process is particularly effective for recovering precious metals like gold and silver, as well as industrial metals such as copper, cadmium, and zinc. The article details the technical aspects of metal deposition, discusses practical applications, and provides a specific example of copper electrowinning to illustrate the process's industrial implementation.
Introduction to Electrowinning
The electrowinning process is a relatively traditional method to recover waste metals through the rinse system and at the same time reduce waste water generation and chemical discharge. The most common metals recovered using electrowinning are gold, silver, copper, cadmium and zinc due to their relative value.
System Components and Operation
System Components and Operation Electrowinning is an electrolytic technology used to recover metals from electroplating rinse waters. Although electrowinning has traditionally been used only for metal recovery, its application in a well-designed and controlled rinse system can significantly reduce rinse water use, wastewater generation, and chemical discharge. An electrowinning unit has three main components: (1) an electrolytic cell, (2) a rectifier, and (3) a pump.

Figure 1: Electrowinning Rectifier and Electrolytic Cell
The electrolytic cell and rectifier are shown in Figure 1. An electrolytic cell is a tank in which cathodes and anodes are typically arranged in alternating order (see Figure 2). The cathodes and anodes are attached to their respective bus bars, which supply the electrical potential to the unit. The electrolytic cell may include features to improve rinse water circulation within the cell, such as a flow disperser or air spargers.
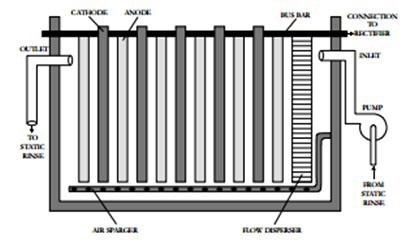
Figure 2: Cross-section of Electrowinning Unit
Process Mechanics
When an electrowinning unit is in operation, the electrical potential applied to the electrodes causes dissolved metals and other positively charged ions to migrate toward and plate onto the cathodes. As metals deposit on the cathodes, the metal buildup decreases the deposition rate. When the metal deposition rate is no longer sufficient, cathodes are removed from the electrolytic cell for on-site or off-site metal recycling. In some cases, recovered metals are pure enough to be reused in process baths. As metals are chemically reduced at the cathodes, other rinse water components are oxidized at the anodes. If cyanide is present, it is oxidized to cyanate and then to carbon dioxide and nitrogen.
Metal Recovery Applications
Electrowinning is most commonly used to recover gold, silver, copper, cadmium, and zinc. Gold and silver are the most successfully recovered metals because of their high electropotential. Chromium is the only metal commonly used in electroplating that is not recoverable by electrowinning. Nickel recovery is possible, but the process is very pH-sensitive as the pH must be maintained within a small range for metal deposition to occur. Some fluoroborate-containing solutions, such as tin and tin-lead solutions, can corrode certain anode materials. Most etchant solutions dissolve metals off the cathodes as quickly as they are deposited.
Copper Electrowinning Example
During the electrowinning process, copper ions, which are contained in an EW electrolyte solution, are plated onto stainless steel blank sheets or copper starter sheets. When the stainless steel blank sheets are submersed into tanks filled with an electrolyte solution, copper essentially "grows" on the blanks via electrolysis where the cross current creates a solid, 2-4 inch thick piece of pure copper, or cathode.
Technical Reactions
In electrowinning, metal is deposited from solution onto cathode blanks by application of a direct electrical current according to equation (1). In a competing reaction, hydrogen ions are reduced to gaseous hydrogen according to (2). As the concentration of metal ions in solution is lowered the potential of (1) approaches that of (2) and the majority of the current is consumed by the hydrogen evolution process.
Oxygen evolves simultaneously from insoluble anodes, according to equation (3) below.
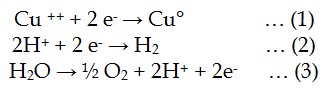
Figure 3: Electrowinning reactions
Process Considerations
For the most part, electrowinning cells utilize a basic design comprising alternating flat sheet cathodes and anodes. As metal is plated onto the cathode, the stagnant liquid film adjacent to the cathode surface tends to become depleted in metal ions. The current density, which determines the rate of metal deposition, must exceed the rate at which metal ions diffuse through this film to the cathode. If the current density is too high, the concentration of metal ions in the film will be excessively depleted producing a condition known as "concentration polarization". This results in an adverse effect on the current efficiency as well as the quality of the deposit, as a result of hydrogen evolution.
Read more
Find Instantly Thousands of Heat Treatment Diagrams!
Total Materia Horizon contains heat treatment details for hundreds of thousands of materials, hardenability diagrams, hardness tempering, TTT and CCT diagrams, and much more.

Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.