Electrowinning Process: Part Two
Abstract
This article explores advanced electrowinning methods for precious metal recovery, with particular focus on gold extraction from aurodicyanide solutions derived from loaded carbon elution. The electrochemical reactions governing gold electrowinning are analyzed, including the preferential deposition of gold over copper under specific conditions. Additionally, the paper examines alternative approaches such as the Ezinex process for zinc electrowinning and presents research on nickel recovery from ammonia-ammonium chloride solutions, highlighting temperature and potential effects on current efficiency. These optimized electrowinning techniques offer significant economic advantages in metal recovery operations.
Gold Recovery Through Electrowinning
Electrowinning represents an effective method for recovering gold from concentrated aurodicyanide solutions produced during loaded carbon elution. This process is particularly valuable when the plated gold can be easily removed using high-pressure water sprays, making it economically advantageous when processing gold-plated components.
The following electrochemical reactions are crucial during the gold electrowinning process (E values quoted for metal ion concentrations of 10-4 mol.dm-3, NaCN concentration of 0.2%, and NaOH concentration of 2%):
Au(CN)2- + e- ↔ Au + 2CN- [1]
E = -0.672 VSHE
O2 + 2H2O + 4e- ↔ 4OH- [2]
E = 0.419 VSHE
2H2O + 2e- ↔ H2 + 2OH- [3]
E = -0.809 VSHE
Cu(CN)32- + e- ↔ Cu + 3CN- [4]
E = -0.760 VSHE
Gold, present in solution as aurodicyanide (Au(CN)2-), reduces to metallic gold according to Reaction [1] at potentials more negative than the reversible potential. Competing with gold deposition is oxygen reduction in alkaline solutions (Reaction [2]), which occurs because the electrolyte typically becomes saturated with oxygen due to evolution at the anode. Hydrogen evolution (Reaction [3]) occurs at significant rates at potentials more negative than -0.96 VSHE when operating at pH values above 10.
At potentials significantly more negative than -0.96 VSHE, hydrogen evolution consumes a substantial proportion of the cathodic current due to kinetic control. Meanwhile, the primary anodic reaction involves water oxidation to oxygen, as indicated in Reaction [2].
Metal Selectivity and Co-Deposition Challenges
Reaction [4] shows the reduction of cuprous cyanide (Cu(CN)32-) to metallic copper. The more negative potential required for copper reduction compared to gold reduction indicates that gold should plate preferentially under standard conditions. However, copper may co-deposit with gold when high overpotentials are applied or when copper concentration significantly exceeds gold concentration.
Alternative Electrowinning Processes
The Ezinex process, commercialized in Italy, offers an alternative approach for zinc electrowinning from zinc ammonia chloride solutions. In this process, zinc electrodeposits at the cathode while ammonia oxidizes at the anode. The system employs titanium cathodes for zinc plating and graphite electrodes for ammonia oxidation.
Nickel electrodeposition from ammonium chloride solutions potentially provides advantages over traditional acid sulfate or acid chloride electrowinning by reducing hydrogen production. The nickel deposition reaction at the cathode can be expressed by Reaction [5]:
Ni(NH3)2Cl2 + 2e- → Ni0 + 2NH3 + 2Cl- [5]
Advanced Research in Nickel Electrowinning
Cruz-Gaona et al. conducted a fundamental electrochemistry study on cathode processes during nickel electrowinning from ammonia-ammonium chloride solutions. Their research utilized various solution compositions, with a base composition of 0.2 M NiCl2 and 4 M NH4Cl, with variable amounts of NH3 (added as NH4OH).
Cyclic voltammetry analysis of cathode substrates demonstrated that titanium electrodes provided superior deposit properties and electrochemical behavior compared to platinum and glassy carbon electrodes.
The researchers studied the effect of ammonium chloride on the electrochemical behavior of nickel from chloride media (Figure 1). Their findings revealed significant advantages of nickel electrodeposition from ammonium chloride solutions over traditional chloride solutions.
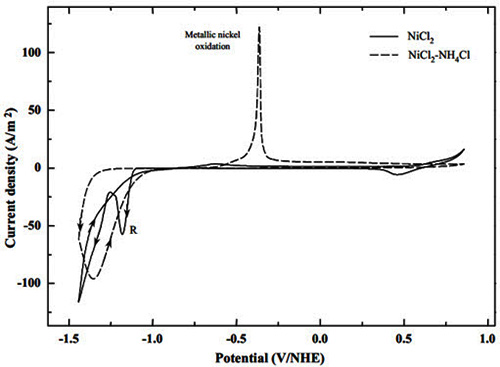
Figure 1: Cyclic voltammetry of 0.2 M NiCl2 and 0.2 M NiCl2-4M NH4Cl using Titanium electrode
The most notable feature was the absence of nickel oxide/hydroxide formation (peak A) typically observed in chloride media. This absence results from the formation of nickel complexes and proton stabilization by ammonia, potentially leading to higher current efficiency in nickel electrowinning from ammonium chloride solutions.
Temperature and Potential Effects on Electrowinning Efficiency
Voltammetry and chronopotentiometry techniques were used to determine the effects of ammonia concentration and system temperature. The research identified two simultaneous processes during electro-reduction: nickel reduction and hydrogen evolution.
The researchers further analyzed current density and current efficiency at various temperatures and potentials to understand the phenomena involved in nickel electrowinning (Figure 2). Their data showed that current density increases with temperature, though the relationship between applied potential and current density did not follow a consistent pattern across all temperatures.
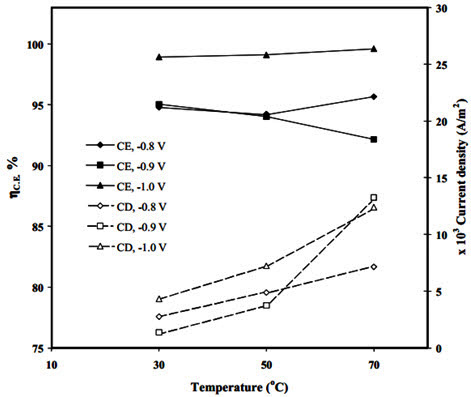
Figure 2: Effect of temperature and applied potential on current efficiency of nickel electrowinning
At 30°C and 50°C, minimum current density occurred at -0.9V, while at 70°C, maximum current was observed at this same potential. These results confirm the presence of at least two competing processes during nickel electrowinning, with the dominant process depending on the applied potential.
Current efficiency (C.E.) studies further clarified these processes. At -0.9V, C.E. decreased with increasing temperature, while at -1.0V, C.E. increased with temperature. This suggests that hydrogen evolution dominates at -0.9V, reducing efficiency as temperature rises. Conversely, nickel electrodeposition becomes favorable at -1.0V and improves with increased temperature, resulting in higher current efficiencies.
Read more
Find Instantly Precise Material Properties!
Total Materia Horizon contains physical, thermal and electrical properties for hundreds of thousands of materials, for different temperatures, and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.