Marine Stainless Steel Applications
Abstract
Marine environments present exceptional challenges for stainless steel applications due to aggressive seawater conditions. While stainless steel offers superior rust resistance compared to brass, bronze, or galvanized steel, even advanced grades from AISI 316 to 6Mo and superduplex materials face corrosion challenges in marine environments. This article examines stainless steel performance in marine applications, focusing on localized corrosion mechanisms including pitting and crevice corrosion, stress corrosion cracking, and galvanic corrosion. Microbiologically induced corrosion (MIC) and biofilm formation further complicate material selection. The discussion covers performance improvement strategies including cathodic protection systems, specialized steel grades like NSS445M2, and proper material selection criteria. Understanding PREN values and implementing appropriate protection methods are essential for successful marine stainless steel applications in industrial facilities concentrated around seaports.
Introduction to Marine Stainless Steel Challenges
Marine applications present some of the most demanding environments for materials selection and performance. Stainless steel has emerged as the preferred material for marine applications because it demonstrates superior rust resistance compared to traditional materials such as brass, bronze, or galvanized steel. However, it has become widely recognized that stainless steels ranging from AISI 316 through 6Mo and superduplex grades do not consistently provide adequate resistance to seawater corrosion.
The reality of marine stainless steel applications reveals that even high-performance alloys can experience significant corrosion challenges. Crevice corrosion and pitting corrosion may develop within relatively short service periods. A notable example involves a 25Cr07Ni super duplex tubular heat exchanger in a marine vessel that exhibited crevice corrosion within just six months of service, demonstrating the aggressive nature of marine environments.
Natural seawater conditions contribute significantly to corrosion acceleration through biofilm development on metal surfaces. These biofilms consistently promote increased water corrosivity, leading to microbiologically induced corrosion (MIC) that frequently occurs in seawater environments. Additionally, galvanic corrosion represents a major operational concern in marine applications.

Figure 1: Marine environment of stainless steels
The growing importance of materials selection in marine environments reflects the worldwide trend toward concentrating major industrial facilities around seaports. This strategic positioning aims to reduce transportation costs while increasing cooling capacity, making marine stainless steel performance optimization increasingly critical for industrial success.
Understanding Localized Corrosion in Marine Environments
Stainless steel corrosion in marine environments never occurs uniformly across the material surface. Instead, corrosion manifests as localized phenomena, specifically pitting corrosion and crevice corrosion. Biofilm formation frequently promotes these localized corrosion mechanisms, creating conditions that accelerate material degradation beyond normal seawater exposure effects.
Crevice corrosion represents a particularly significant challenge in marine environments due to seawater's characteristically low electrical resistivity, approximately 0.35 Ohm•m. This low resistivity creates conditions where even 6% Mo stainless steel can experience crevice corrosion at temperatures as low as 30°C. The aggressive nature of these conditions necessitates careful consideration of material selection and design parameters.
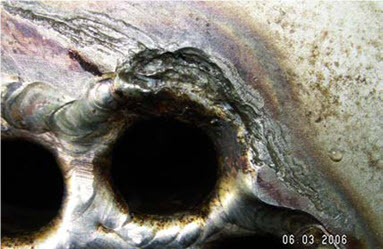
Figure 2: Crevice corrosion in seawater cooler
Pitting corrosion resistance can be evaluated using the Pitting Resistance Equivalent Number (PREN), which provides a quantitative assessment of material performance. The PREN formula is expressed as:
PREN = %Cr + 3.3%Mo + X%N
Where X equals 16 for duplex stainless steels and 30 for austenitic stainless steels. Higher PREN values indicate superior pitting resistance, making this calculation essential for marine material selection.
Chlorinated seawater below 25°C typically does not cause pitting corrosion in duplex stainless steel 2205 and alloys with higher PREN values, providing guidance for temperature-dependent material selection in marine applications.
Stress Corrosion Cracking in Marine Applications
Stress corrosion cracking presents temperature-dependent risks for marine stainless steel applications. Stainless steel grades 304 and 316 become susceptible to chloride cracking at temperatures exceeding 60°C when oxygen is present. Interestingly, produced water from oil or gas production operations does not typically cause stress corrosion cracking, even at elevated temperatures, due to reduced oxygen content.
Duplex stainless steel and 6% Mo grades demonstrate significantly improved resistance to stress corrosion cracking phenomena. However, extreme conditions combining high temperatures, elevated stresses, and cold deformation can still trigger cracking in these advanced materials.
External stress corrosion cracking can occur from outside surfaces, with longitudinal welded pipes at higher temperatures showing particular sensitivity to corrosion under insulation. This mechanism requires careful attention to insulation design and maintenance procedures in marine installations.
Galvanic Corrosion Challenges
The low electrical resistivity of seawater that promotes crevice corrosion also strongly accelerates galvanic corrosion between dissimilar metals. Galvanic corrosion has been identified as a major concern for materials performance in marine environments, requiring systematic consideration during design and material selection phases.
A well-documented example of galvanic corrosion involves bronze bearings in ships, where sacrificial zinc anodes must protect steel hulls from galvanic attack. Stainless steel can both suffer from galvanic corrosion and cause galvanic corrosion in other, less noble alloys when improperly coupled in marine environments.
Cathodic Protection Systems for Marine Stainless Steel
Most corrosion problems affecting stainless steel in marine environments can be prevented through proper cathodic protection implementation. Both impressed current systems and sacrificial anode systems can provide effective protection, though sacrificial anode systems typically offer economic advantages and reduced sensitivity to system failures.
The current requirements for stainless steel cathodic protection are significantly lower than those for carbon steel protection. Stainless steel requires only a few mA/m², compared to approximately 25 mA/m² for carbon steel protection. This difference occurs because carbon steel requires protection against uniform corrosion, while stainless steel only needs polarization of approximately 100 mV to maintain potential below the pitting potential within the safe passive region.
Direct connection of sacrificial anodes to stainless steel creates excessive current flow, resulting in rapid anode consumption and hydrogen charging of the stainless steel. Hydrogen charging can lead to hydrogen embrittlement in duplex stainless steel, titanium hydriding, and embrittlement of ferritic/martensitic stainless steels. Controlling anode current through resistors (RCP anodes) or diodes prevents these problems while maintaining effective protection.
Proper cathodic protection design requires careful engineering analysis to ensure optimal performance while avoiding over-protection effects that can damage the stainless steel or accelerate anode consumption.
External Atmospheric Corrosion Considerations
Uncoated 316L piping and tubing in marine atmospheres experience rust formation and pitting corrosion that ultimately leads to system leakages. Pitting corrosion and chloride cracking occur at temperatures above 60°C, particularly under insulation materials when moisture infiltration occurs. These conditions make external coating application essential for piping systems in marine environments.
Material selection for tubing applications typically specifies 6% Mo stainless steel, which provides adequate resistance to external atmospheric corrosion. However, all uncoated 316 materials, including instrument housings and ancillary equipment, will begin rusting in marine atmospheric conditions.
Advanced Marine Stainless Steel Solutions
The Nisshin Steel Co, Ltd has developed steel grade NSS445M2, a ferritic stainless steel engineered with superior anti-rust and weld anti-corrosion characteristics specifically for marine applications. This specialized material targets roofing and facing applications while providing excellent resistance to hot water containing chlorides.
NSS445M2's main composition includes 22Cr-1.2Mo, with strategic additions of Nb, Ti, and Al to reinforce the surface film and improve rust resistance. The alloy design suppresses chromium loss through oxidation during welding operations, preventing weld zone corrosion resistance degradation. Additionally, its thermal expansion coefficient closely matches common steel and remains lower than austenitic stainless steel, making it particularly suitable for long-span roofing applications.
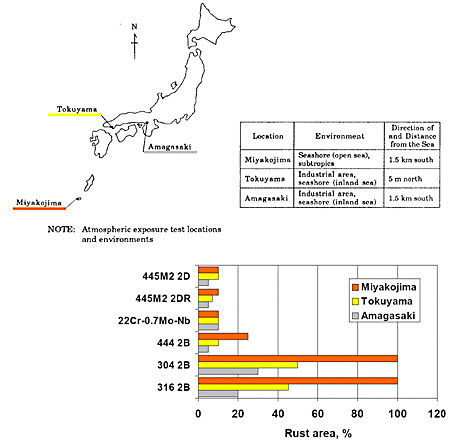


Figure 3: Comparing atmospheric exposure test results
The figure above demonstrates the superior performance of NSS445M2 compared to conventional austenitic grades 304 and 316, as well as ferritic grade 444 in various Japanese marine subtropical environments.
Atmospheric exposure testing conducted in various Japanese marine subtropical areas demonstrates NSS445M2's exceptional performance. The testing results show complete freedom from rusting after two years of exposure, even in highly corrosive environments such as Miyakojima, where conventional stainless steel grades showed significant degradation.
Conclusion
Marine stainless steel applications require comprehensive understanding of corrosion mechanisms and careful material selection to achieve reliable long-term performance. While stainless steel offers advantages over traditional marine materials, successful implementation depends on proper grade selection, cathodic protection design, and recognition of environmental factors that influence corrosion behavior. Advanced materials like NSS445M2 and proper protection systems enable stainless steel to meet the demanding requirements of marine environments while providing economic and operational benefits for industrial facilities located in coastal areas.
Access Precise Properties of Stainless Steels Now!
Total Materia Horizon contains property information for 120,000+ stainless steels: composition, mechanical and physical properties, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.