Applications of Shape Memory Alloys in the medical field
Abstract
This comprehensive review examines the remarkable success of Shape Memory Alloys (SMAs), particularly Nitinol, in medical applications. The unique properties of Nitinol—including shape memory effect, super-elasticity, biocompatibility, and corrosion resistance—have revolutionized medical device design across multiple surgical specialties. The article explores the biocompatibility of Nitinol compared to other implant materials, analyzing its corrosion behavior in the human body and discussing the formation of protective titanium oxide layers that prevent nickel release. Current applications in cardiovascular, orthopedic, and dental fields are presented, along with ongoing research challenges and future developments. As Nitinol continues to enable minimally invasive procedures and innovative implant designs, understanding its complex properties remains crucial for advancing medical applications.
Introduction to Shape Memory Alloys in Medicine
Shape Memory Alloys (SMAs) represent a remarkable success story in the medical applications market, experiencing tremendous growth in usage over recent decades. The significant advances from the surgical perspective have created substantial opportunities for new commercial applications and innovative medical devices.
SMAs possess unique properties that make them exceptionally valuable in medical settings. These include the "shape effect" (both simple and double effect of shape memory), superelasticity, and biocompatibility. The usefulness of these materials can be established based on three key selection criteria: chemical, biological, and mechanical properties. Additionally, SMAs exhibit excellent corrosion resistance, remarkable ductility allowing for easy deformation, great capability to absorb vibration (due to the ease of displacement of internal interfaces), and the capacity to convert heat energy into mechanical energy.
These smart materials have garnered significant attention primarily for their innovative applications in practical settings. The family of shape memory alloys represents arguably the first well-known and widely used smart materials in medical applications.
Biocompatibility of Nitinol in the Human Body
The human body presents a challenging electrochemical environment for implants. Bodily fluids create an aggressive corrosion environment, consisting primarily of an aerated solution containing 0.9% NaCl, along with minor amounts of other salts, organic compounds, serum ions, proteins, and cells—all of which may modify local corrosion effects.
High acidity levels in certain bodily fluids are particularly hostile to metallic implants. Understanding the direct effects of individual components within an alloy is crucial, as these elements can dissolve in the body due to corrosion, potentially causing local and systemic toxicity, carcinogenic effects, and immune responses. The cytotoxicity of elementary nickel and titanium has been extensively researched, especially nickel, which is known to be a toxic agent and allergen. At high concentrations, nickel exhibits toxic effects on soft tissue structures and appears harmful to bone structures, though substantially less so than cobalt or vanadium, which are also routinely used in implant alloys. Experiments with toxic metal salts in cell cultures have demonstrated decreasing toxicity in the following order: Co > V > Ni > Cr > Ti > Fe.
Titanium, on the other hand, is recognized as one of the most biocompatible materials due to its ability to form a stable titanium oxide layer on its surface. Under optimal conditions, titanium is capable of excellent osteointegration with bone and can form a calcium phosphate-rich layer on its surface (Figure 1), very similar to hydroxyapatite, which further prevents corrosion. Another advantageous property is that if the protective layer becomes damaged, the titanium oxides and calcium phosphate layer can regenerate.
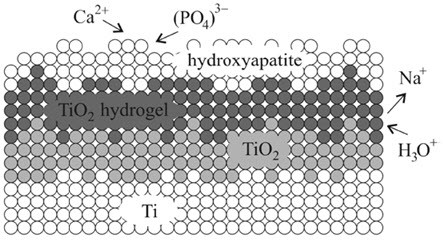
Figure 1: Formation of hydroxyapatite layer on titanium oxide film
The properties and biocompatibility of Nitinol have their own unique characteristics, distinct from those of nickel or titanium alone. In vitro biocompatibility studies examining cellular tolerance and cytotoxicity have been performed using various cell culture models. When human monocytes and microvascular endothelial cells were exposed to pure nickel, pure titanium, stainless steel, and Nitinol, results showed that Nitinol released higher concentrations of Ni²⁺ ions in human fibroblast and osteoblast cultures. Interestingly, this release did not significantly affect cell growth.
Nitinol's Properties and Medical Applications
Nitinol, a group of nearly equiatomic nickel-titanium (NiTi) alloys, has gained widespread recognition and acceptance for medical use. Its exceptional properties—shape memory, superelasticity, and high wear resistance—have enabled the development of innovative instrumentation and implants across diverse surgical fields, from orthopedics to vascular interventions.
The excellent malleability and ductility of Nitinol allow it to be manufactured in various forms including wires, ribbons, tubes, sheets, and bars, providing a broad spectrum of opportunities for medical applications. This versatile biomaterial has proven particularly suitable for minimally invasive procedures, which are often performed in outpatient settings and benefit patients whose health or age status would preclude major "open" surgeries. Additionally, Nitinol has shown great promise in treating younger populations, especially children with congenital defects.
More than thirty years ago, when the first study on Nitinol implantation was completed, Castleman (1976) predicted that while the development path for Nitinol as a biomaterial might be lengthy, its future looked promising. Today, we are witnessing that future. Since its discovery in 1962 at the U.S. Naval Ordnance Laboratory, Nitinol's journey has not always been smooth. Although American in origin, Nitinol quickly established roots globally, with researchers from both Western and Eastern countries contributing to its maturation as a biomaterial: new shape memory features were discovered, innovative multi-component and porous shape memory alloys were developed, and novel surgical techniques utilizing Nitinol's potential were elaborated.
Research Challenges and Future Developments
In vitro and in vivo studies in animal and human models have consistently demonstrated that Nitinol is biocompatible and efficacious as a biomaterial. However, improperly treated Nitinol will invariably present problems with nickel release. To avoid complications related to nickel release and stent metal failures, both the surface and bulk properties of the alloy require careful scrutiny.
The chemical heterogeneity of Nitinol must be acknowledged with respect to medical applications, and the specifics of particulate size and distribution need to be better understood to improve corrosion resistance and fatigue life. Contrary to intuition, the approach that "the thicker the surface oxide layer, the better protection against corrosion and nickel release" is not applicable to superelastic Nitinol. Surface coatings and modifications with various energy sources have not demonstrated significant improvements in Nitinol biocompatibility. Research has shown that the NiTi matrix alloy itself is not a primary reservoir for nickel release; rather, nickel-enriched interfaces formed during heat treatments (especially nickel in a non-oxidized, elemental state) constitute such sources.
The immunostimulatory effect of NiTi, which may be inferred from in vitro and in vivo studies, merits further in-depth investigation. A fascinating aspect of bare Nitinol surfaces relates to the possibility of manipulating Nitinol thrombogenicity—a characteristic of great interest for both applications requiring non-thrombogenic surfaces (like stents) and those benefiting from highly thrombogenic surfaces (such as defect closures and orthopedic/osseogenic implants).
To enhance both immediate response to implantation and long-term implant performance, it is essential to understand the specifics of plasma protein and platelet interactions with Nitinol surfaces. Deeper insights are needed into several aspects of Nitinol surfaces:
- The electronic structure of Nitinol surface oxides, their conductivity and reactivity, nanostructure and defects, surface charge, and oxide stoichiometry
- Their fracture mechanics, microstructure, and compositions
- Studies of explanted devices
Although Nitinol has been in clinical use for more than 40 years, important features continue to be discovered as scientists work to comprehend its complexity.
Biocompatibility and Corrosion Studies
Metal ion release studies have revealed very low concentrations of nickel and titanium released from Nitinol, leading researchers to conclude that the material is not genotoxic. For in vivo biocompatibility assessment, various experiments have been conducted on animals. Several in vivo studies over the past decade have disclosed no allergic reactions, no traces of alloy constituents in surrounding tissues, and no corrosion of implants. Studies examining rat tibiae response to NiTi, compared with Ti-6Al-4V and AISI 316L stainless steel, showed that while the number and area of bone contacts was lower around NiTi implants, the thickness of these contacts was equal to that of other implants. Normal new bone formation was observed in rats 26 weeks after implantation. The favorable biocompatibility results of NiTi are attributed to implants being covered by a titanium oxide layer, with only minimal traces of nickel exposure.
The corrosion resistance of SMAs has also been studied in vivo using animal models. Plates and stents implanted in dogs and sheep for several months were examined under microscope after removal, with pitting identified as the predominant form of corrosion. This led to the development of surface treatments and coatings, which considerably improved corrosion resistance by reducing pitting diameter from approximately 100 μm to only 10 μm in some cases.
Medical Applications of Shape Memory Alloys
The trends in modern medicine increasingly favor less invasive surgical methods, which are typically performed through small, leak-tight portals into the body called trocars. Medical devices made from SMAs utilize a different physical approach that enables them to pull together, dilate, constrict, or push apart, making previously difficult or problematic surgical tasks quite feasible. The unique properties of SMAs are therefore utilized in a wide range of medical applications, as illustrated in Figure 2.
Cardiovascular Applications
Stents represent the most rapidly growing cardiovascular SMA application. These cylindrical mesh tubes are inserted into blood vessels to maintain the inner diameter and prevent blockage. They were developed in response to limitations of balloon angioplasty, which often resulted in repeated blockages in the treated area. Ni-Ti alloys have become the material of choice for superelastic self-expanding (SE) stents used in treating superficial femoral artery disease.
The Simon Inferior Vena Cava (IVC) filter was the first SMA cardiovascular device. It is used for blood vessel interruption to prevent pulmonary embolism through placement in the vena cava. The Simon filter effectively traps blood clots traveling in the bloodstream, allowing them to dissolve naturally over time. The device is constructed from SMA wire curved similarly to an umbrella, creating a trap for circulating clots.
The Septal Occlusion System is indicated for patients with complex ventricular septal defects (VSD) of significant size that warrant closure. It is particularly valuable for patients considered high-risk for standard transatrial or transarterial surgical closure based on anatomical conditions or overall medical status. The system consists of two primary components: a permanent implant constructed of an SMA wire framework with attached polyester fabric, and a coaxial polyurethane catheter specifically designed to facilitate attachment, loading, delivery, and deployment to the defect.
Surgical instruments such as guide wires, dilatators, and retrieval baskets exploit the excellent kink resistance of SMAs. Open heart stabilizers, similar to steerable joint endoscopic cameras, are used to prevent regional heart movements during bypass operations. Another application of SMAs' unique properties—constant force and superelasticity—in heart surgery is the tissue spreader used to separate fatty tissue surrounding the heart.
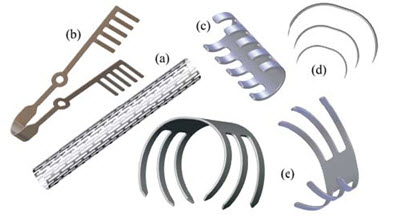
Figure 2: Examples of nitinol medical devices
Orthopedic Applications
In general, conventional orthopedic implants far exceed other SMA implants in weight and volume. They are used as fracture fixation devices (which may or may not require removal) and as joint replacement components. The similarity between bone and nitinol in stress-strain characteristics makes nitinol an ideal material for bone fixation plates, nails, and other trauma implants.
Shape memory fixators represent an advancement by applying the necessary constant force to accelerate fracture healing. The SMA embracing fixator consists of a body and sawtooth arms. New SMA inter-locking intramedullary nails offer numerous advantages compared to traditional designs. For example, when cooled, SMA inter-locking nails can be inserted into a cavity; after guiding nails are extracted, body heat causes the nails to bend into a preset shape, applying constant pressure in the axial direction of the fractured bone. The shape memory effect is also utilized in surgical fixators made from wire.
The SMA Patellar Concentrator was specifically designed to treat patellar fractures. This device exerts continuous compression for the fixation of fractured patella, promoting optimal healing conditions.
Dental Applications
Dentists employ various devices made from SMA for different purposes (Figure 3). NiTi-based SMA materials perform exceptionally well under high strain conditions in strain-controlled environments, as demonstrated with dental drills for root canal procedures. The primary advantage of these drills is their ability to withstand bending to large strains while still accommodating high cyclic rotations. Superelastic SMA wires have found widespread use as orthodontic wires. More recently, a specialized fixator for mounting bridgework has been developed, further expanding dental applications of this versatile material.
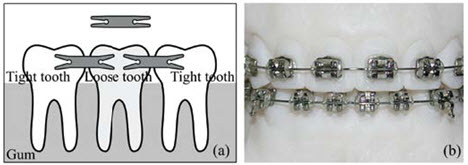
Figure 3: Dental applications of nitinol
Znajdź natychmiast tysiące wykresów obróbki cieplnej! Total Materia Horizon
Total Materia Horizon zawiera szczegóły obróbki cieplnej setek tysięcy materiałów, wykresy hartowności, odpuszczania twardości, wykresy TTT i CCT i wiele więcej.

Uzyskaj BEZPŁATNE konto testowe w Total Materia Horizon i dołącz do społeczności ponad 500 000 użytkowników z ponad 120 krajów.