Sacrificial Anodes: Part One
Abstract
Sacrificial anodes are specifically designed to act as corrosion ‘decoys’ for materials which require protection from a variety of corrosive forces.
Typically with more negative electrochemical potential sacrificial anodes for steel can include zinc, aluminum and magnesium based on their position in the galvanic series.
Sacrificial Anodes are highly active metals that are used to prevent a less active material surface from corroding. Sacrificial Anodes are created from a metal alloy with a more negative electrochemical potential than the other metal it will be used to protect. The sacrificial anode will be consumed in place of the metal it is protecting, which is why it is referred to as a "sacrificial" anode.
To understand the action of sacrificial anodes for cathodic protection it is necessary to have in mind the galvanic series of metals. The galvanic series for a few selected metals in sea water is shown in Table 1.

Table 1: Galvanic series of some metals in sea water
It is evident that when the tendency for metal to go into solution as metal ions increases (leaving an excess of electrons on the metal surface), i.e.
M > M+ + e ... (1)
the metal becomes more electronegative. Thus, since zinc, aluminum and magnesium are more electronegative than steel they are increasingly able to supply electrons to the more electropositive steel when in electrical contact in water, and will effect cathodic protection of the steel surface. Clearly, if steel was coupled to copper in sea water, steel would supply electrons to copper which would become cathodically protected, and the corrosion of the steel would be enhanced. The principle of cathodic protection is well explained by the Wagner-Traud mixed potential theory.
According to this theory, any corrosion process can be divided into two or more oxidation and reduction partial reactions with no net accumulation of electric charge during the process. The corrosion reactions occurring in aluminum in an aqueous medium are shown in Equations 2–4:
Anodic reaction:
Al → Al3+ + 3e− (Aluminum dissolution) … (2)
Cathodic reactions:
O2 + 2H2O + 4e− → 4OH– (Oxygen reduction on Al in neutral or basic solution) … (3)
O2 + 4H+ + 4e− → H2O (Oxygen reduction on Al in acid solutions) … (4)
Corrosion is initiated only when both the anodic and cathodic reactions occur simultaneously. The total rate of oxidation must equal the total rate of reduction in any system. In Figure 1, the relationship between the anodic and cathodic partial corrosion currents for Al has been shown, using mixed potential theory and kinetic equations.
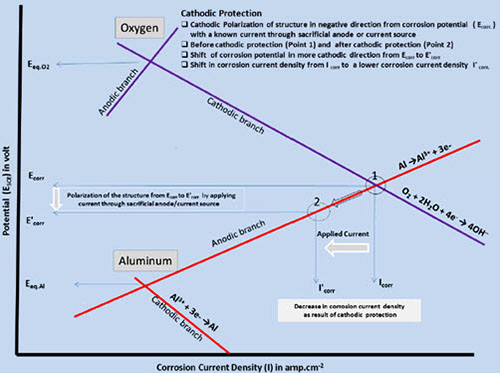
Figure 1: Evans diagram explaining the principle of cathodic protection
As shown in Figure 2, polarization of the cathode in a negative direction from the corrosion potential decreases the corrosion rate. By polarizing the system from Ecorr to E’corr with a known applied current through the sacrificial anode or direct current source, the corrosion current density decreases from Icorr to I'corr.
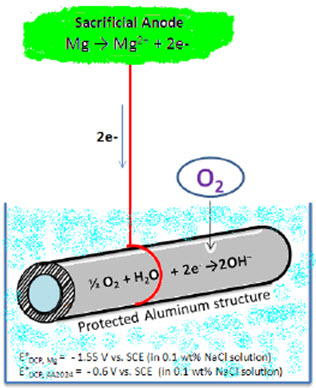
Figure 2: Schematic showing cathodic protection methods using sacrificial anode
For complete inhibition of corrosion processes, it is necessary to polarize the metal to its reversible potential EAl/Al 3+. The applied current at EAl/Al 3+ potential is termed as the protection current.
Read more
Access Precise Corrosion Properties Now!
Total Materia Horizon contains corrosion behaviour and property information for hundreds of thousands of materials, accross more than 2,000 media.

Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.