Magnetic Materials
Abstract
Only three elements out of the total of 106 of periodical table are ferromagnetic at room temperature: Fe, Co and Ni. The combination of these elements together or with others, although not magnetic, creates a wide range of materials, the characteristics of which go from materials able to amplify hundreds of thousands of times the external magnetic field (called “soft”), to materials able to keep their magnetism permanently and to store energy of ten and hundreds of kJ/m3 (called “hard”).
When we speak of “magnetic behavior”, we usually mean ferromagnetic behavior. Actually there are two other types of magnetic behavior: diamagnetic behavior, which is a weak repulsion of a magnetic field and diamagnetism, which is a weak attraction of a magnetic field.
Only three elements out of the total of 106 of periodical table are ferromagnetic at room temperature: Fe, Co and Ni. The combination of these elements together or with others, although not magnetic, creates a wide range of materials, the characteristics of which go from materials able to amplify hundreds of thousands of times the external magnetic field (called “soft”), to materials able to keep their magnetism permanently and to store energy of ten and hundreds of kJ/m3 (called “hard”).
Magnetic moment
The elementary unit in magnetism is the magnetic dipole. The magnetic dipole is as a little magnetized bar, with two poles at the extremities (North and South) that generates a magnetic field around it and is affected to the action of an external magnetic field. The magnetic dipole is indivisible, unlike the electric dipole that is formed by two electrical charges of opposite signs. It means that, in nature, don’t exist magnetic monopoles, the North monopole and the South monopole, but only an indivisible unit.
The quantity that expresses the value of the magnetic dipole is the magnetic moment m, i.e. m is a vector, which module could be expressed in electrodynamical terms as
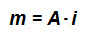
where i is a current that runs in a closed circuit of an area A. The direction is perpendicular to the surface of area A, with the verse given by the right hand rule. Its unit is the A⋅m2.
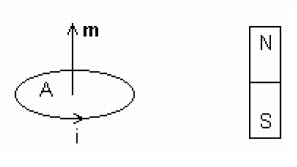
When the magnetic dipole is under the influence of a magnetic field B, it tends to rotate in the direction of the field, to minimize the energy E of interaction:
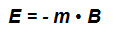
In magnetic substances, the elementary magnetic dipoles are the atoms it selves, which magnetic moments originate from the motion of the electrons around the nucleus and from the electron spins. Thinking to the definition of the magnetic moment, it is evident that, if A is the area of the atomic orbital and i is the current of the electron rotating, the value of m is extremely low.
The macroscopical quantity that is significant for our purposes is the magnetization M, defined as the vectorial sum of all the atomic magnetic moments divided by the volume V of the material:
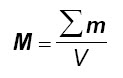
Its unit of measure is the A/m.
The total magnetic induction B (in Tesla) is given by two contributes: M (magnetization, in A/m), that is correlated with magnetic material, and H (magnetic field, in A/m) that is correlated with electrical currents:
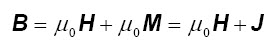
This equation is the fundamental equation of magnetism. μ0 is the vacuum permeability (1.256⋅10-6 H/m). J (=μ0 M) is called magnetic polarization (or intrinsic magnetization), and it is only a convenient way to express the magnetization of a material in tesla.
The different ways with which magnetic moments react to an external magnetic field give different categories of magnetic materials:
- Diamagnets: materials that weakly magnetize themselves in the opposite verse with regard of the external field;
- Paramagnets: materials that weakly magnetize themselves in the same verse with regard of the external field;
- Ferromagnets: materials that strongly magnetize themselves in the same verse with regard of the external field;
Properties of magnetic materials
Saturation magnetization is a measure of the magnetic field a material can produce when all atomic magnets are aligned - how "magnetic" it is Coercivity is the reverse magnetic field required to reduce the net magnetization to zero - how "permanent" it is ("soft" = low coercivity, "hard" = high coercivity).
Other properties often quoted are the permeability of soft materials, which varies inversely with coercivity, and the energy product of hard materials, related to area of "hysteresis loop" and depends on both coercivity and saturation magnetization.
Applications
Hard (permanent) magnetic materials are used in applications requiring a steady magnetic field. Recent increases in coercivity (resistance to demagnetization) and energy product (dependent on both coercivity and saturation magnetization, and related to the area within the hysteresis loop) have greatly increased the use of permanent magnets in modern technology. The widest applications are in motors, speakers, and sensors.
At least as important have been increases in coercivity by even greater factors, which allow a greater flexibility of magnet shape. Low-coercivity steel magnets required long bar magnets or horseshoe shapes to minimize demagnetization from reverse fields produced by the north and south poles at the ends of the magnet. Before the advent of alnico magnets in the 1930s, telephone receivers were long and separate from the speakers because they included a long horseshoe-shaped steel magnet.
Find Instantly Precise Material Properties!
Total Materia Horizon contains physical, thermal and electrical properties for hundreds of thousands of materials, for different temperatures, and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.