Corrosion Inhibitors
Abstract
Corrosion inhibitors are specialized chemical substances that protect materials by minimizing or preventing corrosive action when present in small concentrations. These protective compounds serve critical roles in industrial applications, providing temporary protection during storage and transport, as well as localized protection against aggressive phases such as brine accumulation in oil systems. Inhibitor efficiency is quantitatively measured by comparing corrosion rates with and without the inhibitor presence, using the formula P = (w₀ - w/w₀) × 100. Classification systems divide inhibitors into environmental conditioners (scavengers) and interface inhibitors, with subcategories including anodic, cathodic, mixed, and volatile corrosion inhibitors. Each type operates through distinct mechanisms, from oxygen scavenging to protective film formation, enabling tailored protection strategies for specific metal-environment combinations across diverse industrial applications.
Introduction to Corrosion Inhibitor Technology
Corrosion inhibitors represent a fundamental technology in materials protection, serving as chemical guardians that safeguard metals and other materials from destructive environmental interactions. These specialized substances function by dramatically reducing or completely preventing corrosive processes when introduced in carefully controlled, small concentrations to various environments.
The strategic importance of corrosion inhibitor technology extends across numerous industrial sectors, where material degradation poses significant economic and safety challenges. From protecting critical infrastructure components during long-term storage to providing real-time protection in aggressive operating environments, corrosion inhibitors have become indispensable tools in modern materials management.
A corrosion inhibitor operates as a chemical substance that, when added in small concentrations to an environment, effectively minimizes or prevents corrosion processes. These protective compounds are engineered to address diverse protection scenarios, including temporary safeguarding during storage or transportation phases, as well as providing localized protection against specific threats such as aggressive phase accumulation.
Understanding Corrosion Inhibitor Efficiency and Performance
Quantitative Measurement of Inhibitor Performance
The effectiveness of corrosion inhibitors is precisely quantified through standardized efficiency calculations that enable direct performance comparisons across different systems and applications. An efficient inhibitor demonstrates compatibility with its intended environment, provides economic value in application, and produces the desired protective effect when present in small concentrations.
Inhibitor efficiency, represented by the symbol P, is calculated using the fundamental relationship:
P = (w₀ - w/w₀) × 100
In this critical equation, w₀ represents the corrosion rate measured in the absence of any inhibitor, while w denotes the corrosion rate measured in the identical environment after inhibitor addition. This mathematical approach provides a standardized method for evaluating and comparing inhibitor performance across different applications and environmental conditions.
The efficiency calculation enables engineers and materials specialists to make informed decisions regarding inhibitor selection, concentration optimization, and cost-effectiveness analysis for specific protection requirements.
Classification Systems for Corrosion Inhibitors
Fundamental Classification Approach
Inhibitor selection relies heavily on understanding both the target metal and the environmental conditions where protection is required. The systematic classification of corrosion inhibitors provides a framework for matching appropriate protection technologies with specific application requirements.
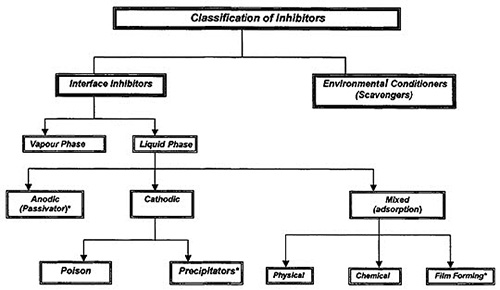
Figure 1: Comprehensive Classification of Corrosion Inhibitors
The primary classification system divides corrosion inhibitors into two major categories: environmental conditioners and interface inhibitors. This fundamental distinction is based on the mechanism through which each category achieves corrosion protection, enabling targeted selection for specific applications and environmental challenges.
Environmental Conditioners and Scavenging Mechanisms
Environmental conditioners, also known as scavengers, represent a sophisticated approach to corrosion control that focuses on modifying the corrosive environment itself rather than directly protecting the metal surface. These inhibitors achieve protection by removing or neutralizing corrosive species present in the surrounding medium.
The scavenging mechanism proves particularly effective in systems where specific aggressive substances drive the corrosion process. By eliminating these corrosive agents from the environment, scavengers create conditions that are inherently less threatening to the protected materials.
In near-neutral and alkaline solution environments, oxygen reduction commonly serves as the primary cathodic reaction driving corrosion processes. Environmental conditioners address this challenge by significantly decreasing oxygen content through specialized scavenging reactions. Hydrazine exemplifies this approach, effectively removing dissolved oxygen and reducing the driving force for corrosion reactions.
The effectiveness of environmental conditioners depends on their ability to selectively target and neutralize specific aggressive species while maintaining compatibility with the overall system chemistry and operational requirements.
Interface Inhibitor Technologies
Protective Film Formation Mechanisms
Interface inhibitors operate through a fundamentally different approach, controlling corrosion by establishing protective films at the critical metal-environment interface. This direct surface protection strategy provides immediate barrier effects that physically separate the metal from aggressive environmental species.
The interface inhibitor category encompasses both liquid-phase and vapor-phase systems, each designed to address specific application requirements and environmental conditions. This versatility enables comprehensive protection strategies that can be tailored to diverse operational scenarios.
Liquid-phase inhibitors are further classified based on their electrochemical interaction mechanisms, specifically whether they primarily inhibit anodic reactions, cathodic reactions, or both electrochemical processes simultaneously. This classification system enables precise matching of inhibitor technology with specific corrosion mechanisms and environmental challenges.
Anodic Inhibitor Systems and Passivation Technology
Anodic inhibitors achieve corrosion protection through the formation of protective oxide films on metal surfaces, creating a fundamental shift in the electrochemical behavior of the protected material. These specialized compounds typically cause substantial anodic shifts in corrosion potential, effectively forcing the metallic surface into the passivation region where corrosion rates become negligible.
The passivation mechanism represents one of the most effective forms of corrosion protection, as it fundamentally alters the surface chemistry to create inherently corrosion-resistant conditions. Anodic inhibitors are sometimes referred to as passivators due to this distinctive mechanism.
Representative examples of anodic inhibitor technology include chromates, nitrates, tungstates, and molybdates. Each of these compound families demonstrates the ability to promote protective oxide film formation while maintaining effectiveness across diverse environmental conditions and metal substrates.
The selection of specific anodic inhibitors depends on factors including the target metal composition, environmental pH, temperature conditions, and compatibility with other system components or processes.
Cathodic Inhibitor Mechanisms and Applications
Cathodic inhibitors provide corrosion protection through two primary mechanisms: directly slowing cathodic reaction rates or selectively precipitating on cathodic areas to limit the diffusion of reducing species to the metal surface. This dual approach enables flexible protection strategies adapted to specific environmental and operational requirements.
The reduction of cathodic reaction rates can be achieved through the use of cathodic poisons, which selectively interfere with the electrochemical processes that drive corrosion. However, the application of cathodic poisons requires careful consideration, as these compounds can potentially increase metal susceptibility to hydrogen-induced cracking. This occurs because hydrogen absorption by the metal can increase during aqueous corrosion processes or cathodic charging conditions.
Alternative cathodic inhibitor strategies focus on oxygen scavenging, where specialized compounds react with dissolved oxygen to eliminate this primary cathodic reactant. Sulfite and bisulfite ions exemplify this approach, combining with dissolved oxygen to form sulfate compounds and effectively removing the oxidizing species from the environment.
The selection between different cathodic inhibitor mechanisms depends on the specific environmental conditions, the acceptable level of hydrogen risk, and the overall system chemistry requirements.
Mixed Inhibitor Systems and Comprehensive Protection
Mixed inhibitors provide comprehensive corrosion protection by simultaneously reducing both cathodic and anodic reactions. These versatile compounds typically function as film-forming substances that promote precipitate formation on metal surfaces, effectively blocking both anodic and cathodic sites through indirect mechanisms.
The effectiveness of mixed inhibitor systems is demonstrated in practical applications such as hard water systems, where elevated calcium and magnesium content reduces corrosivity compared to soft water. This natural protection occurs due to the tendency of dissolved salts to precipitate on metal surfaces, forming protective films that inhibit corrosion processes.
Silicates and phosphates represent the most commonly utilized mixed inhibitor families, offering reliable protection across diverse applications. Sodium silicate, for example, finds extensive use in domestic water softening systems to prevent rust water formation. In aerated hot water systems, sodium silicate provides effective protection for steel, copper, and brass components.
However, the reliability of silicate-based protection depends heavily on pH conditions, requiring careful system monitoring and control. Phosphates similarly require oxygen presence for optimal inhibition effectiveness, limiting their application in certain environmental conditions.
While silicates and phosphates may not provide the exceptional protection levels achieved by chromates and nitrites, they offer significant value in applications where non-toxic additives are essential requirements. This environmental compatibility makes mixed inhibitors particularly valuable in potable water systems and other applications where human exposure or environmental impact must be minimized.
Volatile Corrosion Inhibitor Technology
Advanced Vapor-Phase Protection Systems
Volatile Corrosion Inhibitors (VCI), also designated as Vapor Phase Inhibitors (VPI), represent an advanced protection technology that transports protective compounds through closed environments to corrosion sites via volatilization from a source location. This innovative approach enables protection of components that may be difficult to access through conventional liquid-phase inhibitor application.
The volatilization mechanism allows VCI compounds to reach and protect metal surfaces in complex geometries, enclosed spaces, and multi-component assemblies where direct inhibitor application would be impractical or impossible. This capability makes volatile inhibitors particularly valuable for protecting stored equipment, sealed components, and distributed systems.
Boiler System Applications and Steam Transport
In boiler system applications, volatile basic compounds such as morpholine and hydrazine are transported with steam to provide comprehensive protection throughout the system, including difficult-to-access condenser tubes. These compounds achieve protection through multiple mechanisms, including neutralization of acidic carbon dioxide and shifting surface pH toward less acidic and corrosive values.
The steam transport mechanism ensures that protective compounds reach all wetted surfaces within the boiler system, providing uniform protection that would be difficult to achieve through other inhibitor application methods. This comprehensive coverage is essential for maintaining system integrity and preventing localized corrosion in critical components.
Closed Environment Protection Strategies
In closed vapor spaces such as shipping containers and storage enclosures, volatile solid compounds including salts of dicyclohexylamine, cyclohexylamine, and hexamethylenediamine provide long-term protection for stored components and equipment. These specialized compounds are selected for their optimal volatility characteristics and protective effectiveness.
When volatile inhibitor compounds contact metal surfaces, the protective vapor condenses and undergoes hydrolysis reactions with available moisture to liberate protective ions. This process creates localized protection zones that effectively shield metal surfaces from corrosive attack.
Optimization of Volatile Inhibitor Performance
Effective VCI systems must balance competing performance requirements to achieve optimal protection characteristics. An efficient volatile corrosion inhibitor should provide rapid inhibition response while maintaining long-lasting protection throughout the intended service period.
These dual performance requirements create competing demands on compound volatility characteristics. Rapid protective action requires relatively high volatility to ensure quick transport and surface coverage, while enduring protection requires lower volatility to prevent excessive compound loss and maintain long-term effectiveness.
The optimization of volatile inhibitor systems involves careful selection of compound chemistry, volatility characteristics, and application methods to achieve the desired balance between rapid response and sustained protection. Environmental factors such as temperature, humidity, and air circulation patterns also influence system performance and must be considered in application design.
Advanced Applications and Industry Implementation
Industrial Protection Strategies
Modern industrial applications of corrosion inhibitor technology demonstrate the versatility and effectiveness of these protective systems across diverse operational environments. From petrochemical processing facilities to marine applications, corrosion inhibitors provide essential protection that enables reliable operation and extended equipment service life.
The selection and implementation of appropriate inhibitor systems requires comprehensive understanding of the specific corrosion mechanisms, environmental conditions, and operational requirements for each application. This systems approach ensures optimal protection while maintaining cost-effectiveness and operational compatibility.
Future Developments in Inhibitor Technology
Ongoing research and development in corrosion inhibitor technology focuses on developing more effective, environmentally compatible, and cost-efficient protection systems. Advanced formulations incorporating nanotechnology, smart release mechanisms, and multi-functional additives represent promising directions for future inhibitor development.
The evolution of inhibitor technology continues to address emerging challenges in materials protection while meeting increasingly stringent environmental and safety requirements. These developments ensure that corrosion inhibitor technology will continue to provide essential protection capabilities for future industrial applications.
Conclusion
Corrosion inhibitors represent a critical technology for materials protection across diverse industrial applications. The comprehensive classification system encompassing environmental conditioners, interface inhibitors, and volatile protection systems provides a framework for selecting appropriate protection strategies based on specific application requirements.
Understanding the mechanisms and performance characteristics of different inhibitor categories enables engineers and materials specialists to develop effective protection strategies that balance performance, cost, and environmental considerations. As industrial systems become increasingly complex and performance requirements continue to evolve, corrosion inhibitor technology will remain essential for ensuring reliable operation and extended service life of critical materials and components.
The continued development and optimization of inhibitor systems, combined with improved understanding of corrosion mechanisms and environmental interactions, will enable even more effective protection strategies for future industrial challenges.
Access Precise Corrosion Properties Now!
Total Materia Horizon contains corrosion behaviour and property information for hundreds of thousands of materials, accross more than 2,000 media.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.