Corrosion Inhibitors
Used to protect materials, corrosion inhibitors are chemical substances that when present in small quantities inhibit and either minimize or prevent corrosive action.
Corrosion inhibitors are measured in regards to their efficiency and can be easily calculation by making a direct comparison of corrosion rate with and without the presence of the inhibitor.
A corrosion inhibitor is a chemical substance which, when added in small concentrations to an environment, minimizes or prevents corrosion. Corrosion inhibitors are used to protect metals from corrosion, including temporary protection during storage or transport as well as localized protection, required, for example, to prevent corrosion that may result from accumulation of small amounts of an aggressive phase. One example is brine, in a nonaggressive phase, such as oil. An efficient inhibitor is compatible with the environment, is economical for application, and produces the desired effect when present in small concentrations. Inhibitor efficiency, P, is given as
where W0 is the corrosion rate in the absence of inhibitor, and w is the corrosion rate in the same environment with the inhibitor added.
Classification of Inhibitors
Inhibitor selection is based on the metal and the environment. A qualitative classification of inhibitors is presented in Figure 1. Inhibitors can be classified into environmental conditioners and interface inhibitors.
Environmental Conditioners (Scavengers)
Corrosion can be controlled by removing the corrosive species in the medium. Inhibitors that decrease corrosivity of the medium by scavenging the aggressive substances are called environmental conditioners or scavengers. In near-neutral and alkaline solutions, oxygen reduction is a common cathodic reaction. In such situations, corrosion can be controlled by decreasing the oxygen content using scavengers (e.g., hydrazine).
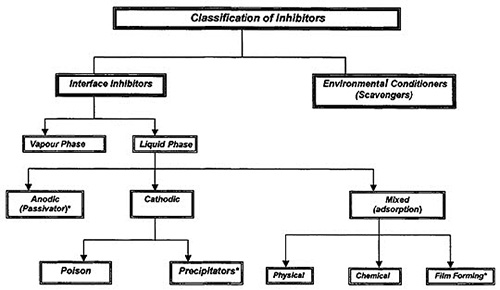
Figure 1: Classification of inhibitors
Interface Inhibitors
Interface inhibitors control corrosion by forming a film at the metal/environment interface. Interface inhibitors can be classified into liquid- and vapor-phase inhibitors.
Liquid-phase inhibitors are classified as anodic, cathodic, or mixed inhibitors, depending on whether they inhibit the anodic, cathodic, or both electrochemical reactions.
Anodic inhibitors
Anodic inhibitors usually act by forming a protective oxide film on the surface of the metal causing a large anodic shift of the corrosion potential. This shift forces the metallic surface into the passivation region. They are also sometimes referred to as passivators. Chromates, nitrates, tungstate, molybdates are some examples of anodic inhibitors.
Cathodic inhibitors
Cathodic inhibitors act by either slowing the cathodic reaction itself or selectively precipitating on cathodic areas to limit the diffusion of reducing species to the surface. The rates of the cathodic reactions can be reduced by the use of cathodic poisons. However, cathodic poisons can also increase the susceptibility of a metal to hydrogen induced cracking since hydrogen can also be absorbed by the metal during aqueous corrosion or cathodic charging.
The corrosion rates can also be reduced by the use of oxygen scavengers that react with dissolved oxygen. Sulfite and bisulfite ions are examples of oxygen scavengers that can combine with oxygen to form sulfate.
Mixed Inhibitors
Mixed inhibitors work by reducing both the cathodic and anodic reactions. They are typically film forming compounds that cause the formation of precipitates on the surface blocking both anodic and cathodic sites indirectly. Hard water that is high in calcium and magnesium is less corrosive than soft water because of the tendency of the salts in the hard water to precipitate on the surface of the metal forming a protective film.
The most common inhibitors of this category are the silicates and the phosphates. Sodium silicate, for example, is used in many domestic water softeners to prevent the occurrence of rust water. In aerated hot water systems, sodium silicate protects steel, copper and brass. However, protection is not always reliable and depends heavily on pH. Phosphates also require oxygen for effective inhibition. Silicates and phosphates do not afford the degree of protection provided by chromates and nitrites, however, they are very useful in situations where non-toxic additives are required.
Volatile Corrosion Inhibitors
Volatile Corrosion Inhibitors (VCI), also called Vapor Phase Inhibitors (VPI), are compounds transported in a closed environment to the site of corrosion by volatilization from a source. In boilers, volatile basic compounds, such as morpholine or hydrazine, are transported with steam to prevent corrosion in the condenser tubes by neutralizing acidic carbon dioxide or by shifting surface pH towards less acidic and corrosive values. In closed vapor spaces, such as shipping containers, volatile solids such as salts of dicyclo-hexylamine, cyclohexylamine and hexamethyleneamine are used.
When these inhibitors come in contact with the metal surface, the vapor of these salts condenses and is hydrolyzed by any moisture to liberate protective ions. It is desirable, for an efficient VCI, to provide inhibition rapidly while lasting for long periods. Both qualities depend on the volatility of these compounds; fast action requiring high volatility while enduring protection requires low volatility.