Duplex Stainless Steels: Part One
Abstract
Duplex stainless steels represent a specialized family of corrosion-resistant alloys featuring a unique microstructure combining austenitic and ferritic grains. These materials contain 18-28% chromium and 4.5-8% nickel, providing properties intermediate between austenitic and ferritic stainless steels. The duplex structure offers exceptional stress corrosion cracking resistance, high strength, and superior chloride attack resistance while maintaining good weldability and formability. This balanced composition results from controlled nickel addition, creating approximately 40-50% austenite in a ferrite matrix. Understanding the formation mechanisms, alloying effects, and potential secondary phases like sigma phase is crucial for optimizing duplex stainless steel performance in demanding industrial applications.
Introduction to Stainless Steel Families
Stainless steel encompasses a comprehensive family of corrosion and heat-resistant steels containing a minimum of 10.5% chromium. Similar to the diverse range of structural and engineering carbon steels that meet varying requirements for strength, weldability, and toughness, stainless steels offer progressively higher levels of corrosion resistance and mechanical strength through controlled alloying element additions..
The controlled addition of specific alloying elements provides unique attributes regarding strength capabilities and environmental resistance characteristics. The available stainless steel grades can be classified into five fundamental families: ferritic, martensitic, austenitic, duplex, and precipitation hardenable steels.
This microstructure-based classification system proves valuable because family members typically exhibit similar physical and mechanical properties. However, properties between different families can vary dramatically. For example, austenitic stainless steels demonstrate non-magnetic behavior, while ferritic and duplex stainless steels exhibit magnetic properties.
Crystal Structure Fundamentals and Atomic Arrangements
The differences between stainless steel families are fundamental at the atomic level, where the arrangement of atoms in ferrite crystals differs significantly from austenite crystal structures.
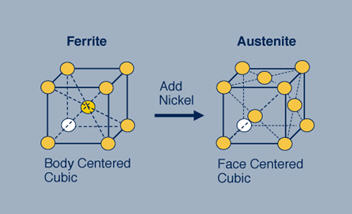
Figure 1: The ferritic stainless steel on the left displays a body-centered cubic (bcc) crystal structure. Adding nickel to this stainless steel changes the structure from bcc to face-centered cubic (fcc), creating the austenitic structure.
In ferritic stainless steel, iron and chromium atoms arrange themselves at cube corners and in the cube center. Conversely, in austenitic stainless steels, the atoms including iron, chromium, and nickel position themselves at cube corners and in the center of each cube face. This seemingly minor structural difference profoundly influences the mechanical and physical properties of these steels.
Comparative Properties of Austenitic and Ferritic Stainless Steels
The fundamental crystal structure differences between austenitic and ferritic stainless steels result in distinct property profiles that determine their respective applications.
Table 1. Select properties of austenitic and ferritic stainless steels, comparing toughness, ductility, weldability, thermal expansion, stress corrosion cracking resistance, and magnetic properties.
| Properties | Austenitic | Ferritic |
| Toughness | Very high | Moderate |
| Ductility | Very high | Moderate |
| Weldability | Good | Limited |
| Thermal expansion | High | Moderate |
| Stress corrosion cracking resistance | Low | Very high |
| Magnetic properties | Non-magnetic | Ferro magnetic |
Due to their superior mechanical properties and fabrication ease, austenitic stainless steels achieve much wider industrial usage than ferritic varieties. Approximately 75% of global stainless steel consumption consists of austenitic grades, while ferritic steels account for about 25%. The remaining families including martensitic, duplex, and precipitation hardenable stainless steels each represent less than 1% of the total market share.
Alloying Element Effects and Microstructure Formation
Beyond nickel, several other elements promote austenitic structure formation and are classified as austenite formers. Conversely, alloying elements that encourage ferritic structure development are termed ferrite formers.
Table 2. Alloying elements formers for stainless steel microstructure
| Ferrite formers | Austenite formers |
| Iron | Nickel |
| Chromium | Nitrogen |
| Molybdenum | Carbon |
| Silicon | Manganese |
| Copper |
Understanding these element interactions enables metallurgists to precisely control microstructure development and achieve desired material properties through strategic alloy composition design.
Duplex Stainless Steel Composition and Structure
Duplex stainless steels achieve their distinctive microstructure through a carefully balanced combination of austenitic and ferritic grains, creating the characteristic "duplex" structure. This unique microstructure results from adding insufficient nickel to produce fully austenitic stainless steel, typically incorporating less nickel than required for complete austenite formation.
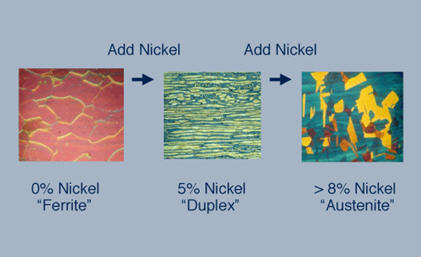
Figure 2: Adding 8% nickel to ferritic chromium stainless steel creates austenitic chromium-nickel stainless steel (Type 304). Using approximately 4-5% nickel produces duplex structure mixing austenite and ferrite, as seen in 2205 duplex stainless steel.
Austenitic-ferritic duplex stainless steels contain increased chromium content ranging from 18% to 28% and reduced nickel content between 4.5% and 8% compared to standard austenitic steels. Many duplex grades incorporate molybdenum as an additional alloying element to enhance specific performance characteristics.
Since the nickel quantity remains insufficient for complete austenitic structure formation, duplex steels develop mixed austenitic-ferritic microstructures that provide balanced properties between purely austenitic and ferritic grades.
Properties and Performance Characteristics
The properties of duplex stainless steels fall between those of austenitic and ferritic steels, offering unique combinations of strength, corrosion resistance, and fabricability. Duplex steels demonstrate exceptional resistance to stress corrosion cracking and chloride ion attack while maintaining good weldability and formability characteristics combined with high mechanical strength..
In the annealed condition, most wrought duplex stainless steels contain approximately 40-50% austenite distributed within a ferrite matrix. During solidification, delta ferrite forms initially, followed by varying amounts of austenite formation as the final material solidifies, depending on the specific composition.
Additional austenite formation occurs through solid-phase transformation during subsequent annealing treatments. Consequently, annealed products typically contain higher austenite percentages than as-cast or as-welded materials. Maintaining sufficient austenite content is essential for achieving satisfactory corrosion resistance and mechanical properties, with optimal austenite levels varying according to service application, alloy composition, and thermal processing history.
Secondary Phase Formation and Sigma Phase Concerns
Additional phases that can form in duplex stainless steels include sigma (σ), chi (χ), R phase, alpha prime (α'), carbides, and nitrides. These phases have been extensively studied through laboratory isothermal heat treatment investigations.
Sigma Phase Characteristics and Formation
Sigma phase represents a hard, brittle intermetallic phase typically containing iron, chromium, and molybdenum in most duplex stainless steel compositions. In these alloys, sigma phase formation generally occurs between approximately 600°C and 950°C, with maximum formation rates occurring between 700°C and 900°C.
Sigma phase typically nucleates at austenite-ferrite grain boundaries and grows into adjacent ferrite regions. Additional austenite often forms in chromium-depleted areas adjacent to sigma phase formations. Elements that stabilize ferrite, including chromium, molybdenum, and silicon, increase sigma phase formation tendency.
On a weight percentage basis, molybdenum promotes sigma phase formation much more effectively than chromium, particularly at elevated temperatures around 900°C. Austenite-forming elements such as nickel or nitrogen can accelerate sigma phase nucleation and growth, although these elements may reduce the total sigma phase volume formed.
The partitioning of alloying elements results in increased concentrations within the phases they stabilize. As nickel or nitrogen stabilize additional austenite, the reduced ferrite volume becomes enriched in chromium and molybdenum. Consequently, while nickel or nitrogen additions may reduce sigma phase formation due to decreased ferrite volume fractions, the effect requires careful consideration in alloy design.
Sigma phase formation can deplete chromium and molybdenum in surrounding areas, reducing overall corrosion resistance. As little as 1% sigma phase may significantly reduce impact toughness, while approximately 10% sigma phase can cause complete embrittlement of duplex stainless steels.
Read more
Access Precise Properties of Stainless Steels Now!
Total Materia Horizon contains property information for 120,000+ stainless steels: composition, mechanical and physical properties, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.