Duplex Stainless Steels: Part One
Abstract
Stainless steel is the name given to a family of corrosion and heat resistant steels containing a minimum of 10.5% chromium. Just as there is a range of structural and engineering carbon steels meeting different requirements of strength, weldability and toughness, so there is a wide range of stainless steels with progressively higher levels of corrosion resistance and strength.
Duplex stainless steels have a mixture of austenitic and ferritic grains in their microstructure; hence they have a "duplex" structure. This effect is achieved by adding less nickel than would be necessary for making a fully austenitic stainless steel.
Stainless steel is the name given to a family of corrosion and heat resistant steels containing a minimum of 10.5% chromium. Just as there is a range of structural and engineering carbon steels meeting different requirements of strength, weldability and toughness, so there is a wide range of stainless steels with progressively higher levels of corrosion resistance and strength. This results from the controlled addition of alloying elements, each offering specific attributes in respect of strength and ability to resist different environments. The available grades of stainless steel can be classified into five basic families: ferritic, martensitic, austenitic, duplex and precipitation hardenable.
The division based on microstructure is useful because the members within one family tend to have similar physical and mechanical properties. However, the properties for one family can be very different from the properties for another family. For example, austenitic stainless steels are non-magnetic, while ferritic and duplex stainless steels are magnetic.
The difference between the families is fundamental on the atomic level. The arrangement of atoms in the ferrite crystal is different from the one in the austenite crystal:
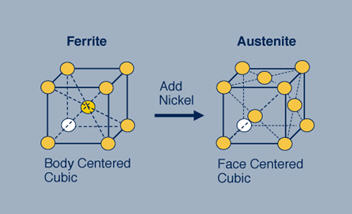
Figure 1: The ferritic stainless steel on the left has a body centered cubic (bcc) crystal structure. By adding nickel to this stainless steel the structure changes from bcc to face centered cubic (fcc), which is called austenitic.
In the ferritic stainless steel, the iron and chromium atoms are arranged on the corners of a cube and in the center of that cube. In the austenitic stainless steels the atoms, here iron, chromium and nickel, are arranged on the corners of the cube and in the center of each of the faces of the cube. This seemingly small difference profoundly affects the properties of these steels.
Table 1: Select properties of austenitic and ferritic stainless steels
| Properties | Austenitic | Ferritic |
| Toughness | Very high | Moderate |
| Ductility | Very high | Moderate |
| Weldability | Good | Limited |
| Thermal expansion | High | Moderate |
| Stress corrosion cracking resistance | Low | Very high |
| Magnetic properties | Non-magnetic | Ferro magnetic |
Because of their good mechanical properties and the ease of fabrication, austenitic stainless steels are much more widely used than ferritic stainless steels. About 75% of all stainless steel used worldwide is austenitic and about 25% is ferritic. The other families, martensitic, duplex and precipitation hardenable stainless steels each represent less than 1% of the total market.
Besides nickel there are other elements that tend to make the structure austenitic. These elements are called austenite formers. Alloying elements that tend to make the structure ferritic are called ferrite formers.
Table 2: Alloying elements formers for stainless steel microstructure
| Ferrite formers | Austenite formers |
| Iron | Nickel |
| Chromium | Nitrogen |
| Molybdenum | Carbon |
| Silicon | Manganese |
| Copper |
Duplex stainless steels have a mixture of austenitic and ferritic grains in their microstructure; hence they have a "duplex" structure. This effect is achieved by adding less nickel than would be necessary for making a fully austenitic stainless steel.
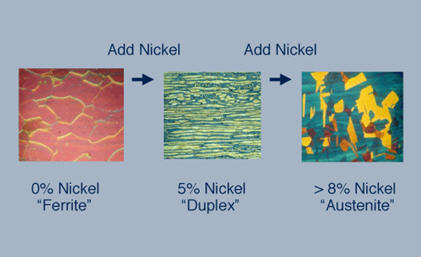
Figure 2: Adding 8% nickel to a ferritic chromium stainless steel makes an austenitic chromium-nickel stainless steel, for example Type 304 stainless steel. If less nickel is added to a chromium steel, about four or five percent, a duplex structure, a mixture of austenite and ferrite, is created as in 2205 duplex stainless steel.
Austenitic-ferritic (Duplex) stainless steels contain increased amount of chromium (18% -28%) and decreased (as compared to austenitic steels) amount of nickel (4.5% - 8%) as major alloying elements. As additional alloying element molybdenum is used in some of Duplex steels. Since the quantity of nickel is insufficient for formation of fully austenitic structure, the structure of Duplex steels is mixed: austenitic-ferritic.
The properties of Duplex steels are somewhere between the properties of austenitic and ferritic steels. Duplex steels have high resistance to the stress corrosion cracking and to chloride ions attack. These steels are weldable and formable and possess high strength.
In the annealed condition, most wrought duplex stainless steels contain about 40-50% austenite in a ferrite matrix. When these materials solidify, σ ferrite forms first. Depending upon the composition, a varying amount of austenite is expected to form as the last material solidifies.
Additional austenite forms by a solid-phase transformation during subsequent annealing. Accordingly, an annealed product is expected to contain more austenite than as-cast or as-welded material. A sufficient amount of austenite must be maintained to provide satisfactory corrosion resistance and mechanical properties. This amount of austenite may vary with the service application and with alloy composition and thermal history.
Additional phases found in duplex stainless steels can include σ, χ, R, α', carbides and nitrides. These phases have generally been studied using isothermal heat treatments in the laboratory.
Sigma Phase
Sigma is a hard, brittle intermetallic phase which is expected to contain iron, chromium and molybdenum in most duplex stainless steels. In these alloys, σ generally can be formed between about 600 and 950°C, with the most rapid formation occurring between 700 and 900°C.
Sigma typically nucleates in the austenite-ferrite grain boundaries and grows into the adjacent ferrite. Often, additional austenite forms in the areas of chromium depletion adjacent to the σ phase. Elements which stabilize ferrite such as chromium, molybdenum and silicon increase the tendency to form the σ phase. On a weight percent basis, molybdenum can promote σ phase formation much more effectively than chromium, particularly at higher temperatures (e.g. about 900°C). Austenite forming elements such as nickel or nitrogen can also accelerate the nucleation and growth of the σ phase, although these elements may reduce the total amount formed.
The alloy elements are portioned, and increased levels of each element tend to be present in the phases they stabilize. As nickel or nitrogen stabilize additional austenite, the reduced amount of ferrite becomes enriched in chromium and molybdenum. As a result, σ phase formed may be reduced by nickel or nitrogen, however, because of the smaller volume fraction of ferrite.
The σ phase can deplete chromium and molybdenum in surrounding areas and reduce resistance to corrosion. As little as about 1% σ phase may reduce impact toughness, while about 10% can cause "complete embrittlement" of duplex stainless steels.
Mehr lesen
Greifen Sie jetzt auf die präzisen Eigenschaften von Edelstahl zu!
Total Materia Horizon enthält Eigenschaftsinformationen für mehr als 120.000 nichtrostende Stähle: Zusammensetzung, mechanische und physikalische Eigenschaften, nichtlineare Eigenschaften und vieles mehr.
Holen Sie sich ein KOSTENLOSES Testkonto bei Total Materia Horizon und schließen Sie sich einer Gemeinschaft von über 500.000 Benutzern aus mehr als 120 Ländern an.