The Nitrocarburizing Process: Part Three
Abstract
Nitrocarburizing represents a critical thermochemical surface treatment process designed to enhance the surface hardness of steel components through controlled nitrogen and carbon diffusion. This comprehensive study examines the effects of nitrocarburizing on AISI 1020 carbon steel and X37CrMoV5-1 hot working tool steel, analyzing microstructure formation, hardness profiles, wear resistance, and corrosion behavior. The process creates a characteristic white layer consisting of iron nitrides (Fe4N and Fe3N) that significantly improves surface hardness, achieving values between 300-1000 HV depending on base material composition. While nitrocarburizing substantially enhances mechanical properties and wear resistance, research indicates potential reduction in corrosion resistance compared to untreated materials, requiring careful consideration for specific industrial applications.
Understanding Nitrocarburizing: Fundamentals and Industrial Applications
Nitrocarburizing represents one of the most effective thermochemical surface treatment processes for enhancing the mechanical properties of steel components. This advanced surface hardening technique combines nitrogen and carbon diffusion to create a hardened surface layer that dramatically improves wear resistance and surface durability. The process finds extensive application in manufacturing critical automotive components including valves, connecting rods, crankshafts, and camshafts, as well as cutting tools and forming dies.
The metallurgical principles of nitrocarburizing center on controlling the formation of a characteristic white diffusion layer through systematic nitrogen dispersion in steel substrates. This process creates distinct phases depending on the base material composition, with iron nitride Fe4N forming in γ-iron matrices up to 6% nitrogen content, while Fe3N phases develop in ε-iron structures accommodating up to 11% nitrogen concentration.
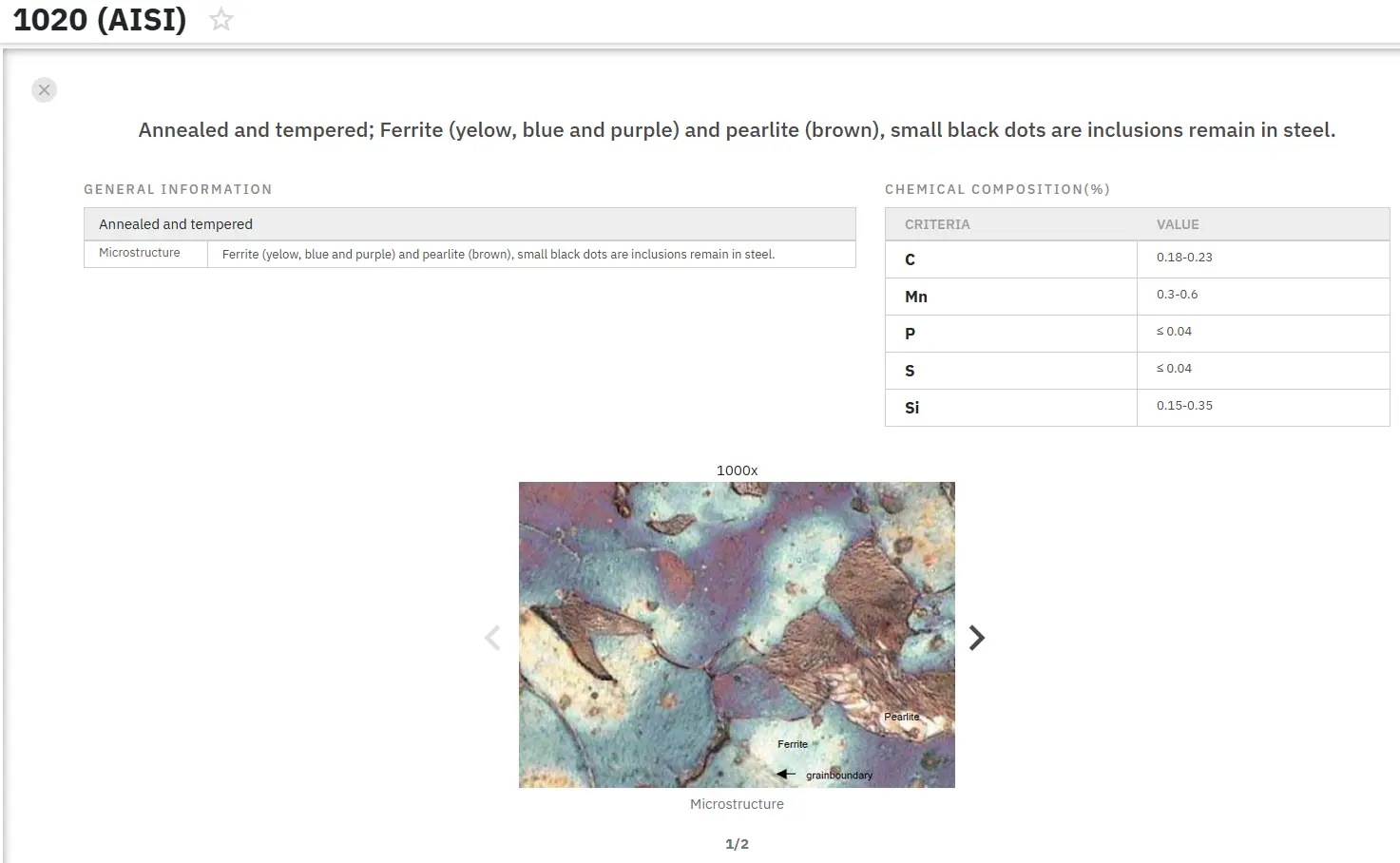
Research conducted by H. A. Ameen and K. S. Hassan on AISI 1020 carbon steel provides valuable insights into the nitrocarburizing effects on material properties. Their investigation utilized 20x10 mm test specimens manufactured according to ASTM standards, employing a sequential treatment process: one hour of liquid nitriding at 550°C followed by liquid carburizing at 925°C for one hour in a saline medium. The study comprehensively analyzed microstructural changes, hardness variations, and corrosion behavior through electrochemical testing in seawater environments.
Microstructural Analysis and Phase Formation in Nitrocarburizing
The nitrocarburizing microstructure formation involves complex metallurgical transformations that determine the final properties of treated components. The white surface layer receives its designation because these phases maintain their characteristic appearance even after etching with Nital solution, distinguishing them from the underlying base material.
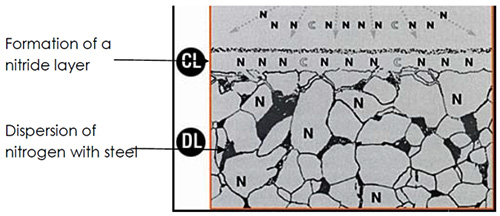
Figure 1: Dispersion of nitrogen in steel
The composition of the surface layer depends significantly on the chemical composition of the base steel. High carbon steels predominantly develop ε-phase structures, while low carbon steels favor γ-phase formation. Detection and characterization of these phases require sophisticated analytical techniques, particularly X-ray diffraction analysis, to accurately identify the crystallographic structures and phase distributions.
The formed surface layer consists primarily of non-metallic phases called nitrides, with their proportions directly influenced by the steel's chemical composition. The presence of alloying elements creates additional complexity as these elements can combine with nitrogen or carbon to form various nitrides or carbides, contributing to the overall hardness and performance characteristics of the treated surface.
Process Parameters and Hardness Characteristics
Nitrocarburizing process parameters typically involve treatment temperatures between 530-600°C, with the depth of the formed layer ranging from 0.2-1 mm depending on the base metal's chemical composition and treatment duration. The hardness achieved through nitrocarburizing varies considerably based on the carbon content of the substrate material. Low carbon steels typically achieve hardness values between 300-400 HV, while high carbon steels can reach impressive hardness levels of 700-1000 HV.
The research conducted by A. Ciski and T. Babul on X37CrMoV5-1 hot working tool steel demonstrates the practical application of nitrocarburizing for tool steel enhancement. Their investigation examined samples subjected to nitrocarburizing at 560°C for treatment durations of 4 and 8 hours, following preliminary heat treatment consisting of austenitizing at 1030°C with nitrogen cooling at 0.6 MPa pressure, followed by double tempering at 525°C for 2 hours and 580°C for 2 hours.
The microstructural analysis revealed the formation of compact, non-etching subsurface layers of iron carbonitrides with thicknesses directly proportional to treatment time. Specimens treated for 4 hours developed layers approximately 2.5 μm thick, while 8-hour treatments produced layers of approximately 5 μm thickness. These white layers demonstrated excellent adhesion to the substrate with minimal porosity in the outer regions, indicating optimal nitrogen and carbon activity during processing.
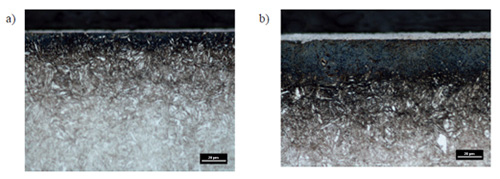
Figure 2: Microstructure of X37CrMoV5-1 steel after 4h (a) and 8h (b) nitrocarburizing process
Hardness Testing and Mechanical Property Evaluation
Comprehensive hardness testing of nitrocarburized surfaces reveals the substantial mechanical property improvements achieved through this treatment. The X37CrMoV5-1 steel specimens demonstrated remarkable hardness values, with 4-hour treatments achieving approximately 796-808 HV0.5 and 8-hour treatments reaching 713-755 HV1. The variation in hardness measurements under different loads may result from the inherent variability in hardness testing or the influence of the extremely hard white layer being penetrated during higher load measurements.
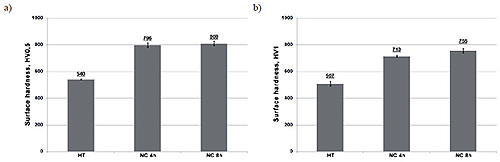
Figure 3: HV0.5 (a) and HV1 (b) hardness of X37CrMoV5-1 steel after nitrocarburizing process
The hardness profile analysis across the cross-section of treated specimens provides crucial information about the effectiveness of the diffusion process and the gradient of mechanical properties from the surface to the core material. This gradient hardness profile is essential for understanding how the treated components will perform under various loading conditions and stress distributions.
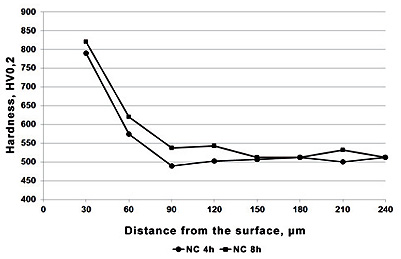
Figure 4: HV0.2 hardness profile at the cross section of X37CrMoV5-1 steel after nitrocarburizing process
Wear Resistance and Impact Strength Considerations
The enhanced wear resistance of nitrocarburized steel represents one of the most significant benefits of this surface treatment technology. Comparative analysis demonstrates that nitrocarburized steel exhibits substantially higher hardness and wear resistance compared to conventionally quenched and tempered materials. Interestingly, the wear resistance characteristics remained relatively consistent regardless of the nitrocarburizing treatment duration, suggesting that even shorter treatment times can achieve optimal wear performance.
However, the impact strength properties showed more significant variation with treatment time. Components subjected to different nitrocarburizing durations demonstrated notably different impact strength values, while hardness variations remained relatively modest. This finding suggests that nitrocarburizing time optimization requires careful consideration of the specific application requirements and the balance between surface hardness, wear resistance, and impact toughness.
The research indicates that a 4-hour nitrocarburizing treatment provides an optimal compromise between various mechanical properties. This duration achieves relatively high surface hardness, excellent cross-sectional hardness distribution, superior wear resistance, and acceptable impact strength characteristics. The resulting diffusion layers confirm the practical applicability of the fluidized bed nitrocarburizing method for X37CrMoV5-1 steel components.
Industrial Applications and Environmental Advantages
The practical applications of nitrocarburizing in industrial manufacturing extend beyond traditional tooling applications. The enhanced surface properties make nitrocarburized components particularly suitable for tools used in plastics processing, especially for polymers in chemical, medical, and food industries. These applications demand not only high wear resistance but also excellent corrosion resistance due to exposure to acids and chlorides formed during thermoplastic decomposition, such as those released from PVC processing.
The environmental benefits of nitrocarburizing provide additional advantages for industrial implementation. The fluidized bed nitrocarburizing method offers significant benefits compared to traditional bath, gas, and plasma techniques. The process requires only a powder mixture with specified chemical composition, eliminating the need for liquid or gaseous technological media. This characteristic substantially reduces equipment operation costs while maintaining environmental safety standards.
The ease of furnace operation and the absence of hazardous liquid or gaseous media make this process particularly attractive for widespread industrial adoption. The method demonstrates excellent environmental compatibility while delivering superior metallurgical results, representing an optimal balance between performance enhancement and environmental responsibility.
Corrosion Resistance Considerations and Future Research
While nitrocarburizing provides exceptional improvements in mechanical properties, the corrosion resistance of nitrocarburized steel requires careful evaluation for specific applications. Research findings suggest that the corrosion resistance of treated specimens may be reduced compared to untreated base materials, particularly when evaluated through electrochemical testing in seawater environments using Tafel equation analysis.
This reduction in corrosion resistance presents an important consideration for component designers and manufacturers when selecting surface treatment options. The trade-off between enhanced mechanical properties and potentially reduced corrosion resistance must be carefully evaluated based on the specific service environment and performance requirements of the application.
Future research directions should focus on developing modified nitrocarburizing processes that can maintain or enhance corrosion resistance while preserving the excellent mechanical property improvements. Additionally, investigation into post-nitrocarburizing treatments that could restore or improve corrosion resistance without compromising the beneficial hardness and wear characteristics would be valuable for expanding the application range of this technology.
Find Instantly Thousands of Metallography Diagrams!
Total Materia Horizon contains a unique collection of metallography images across a large range of metallic alloys, countries, standards and heat treatments.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.