The Caron Process
Abstract
The growing global demand for nickel has heightened interest in processing techniques for the world's nickel reserves. The Caron Process, an effective method for nickel extraction, involves reduction roasting of laterite ores followed by ammonia leaching of the reduced materials. This article examines the principles, operational parameters, and chemical reactions underlying the Caron Process, with particular focus on its application to limonitic laterite ores. While effective for iron-rich limonites, the process faces economic challenges with saprolitic ores containing high magnesium and silicon content. The critical reduction stage, subsequent leaching reactions, and thermodynamic considerations for optimal nickel recovery are analyzed, highlighting both the capabilities and limitations of this important metallurgical technique.
Introduction to Nickel Resources and Extraction Methods
Nickel occurs in nature principally as sulfides and laterites (oxides and silicates). Nickel laterite ores account for approximately 80% of the world's nickel reserves and are becoming increasingly important for global nickel supply as demand has grown in recent decades.
In general, nickel can be extracted from its ores using three main processes:
- Smelting
- High Pressure Acid Leaching (HPAL)
- Caron roast/leach process
The Caron Process: Fundamentals and Applications
The Caron process, which involves reduction roasting of the ore followed by ammonia leaching of reduced ore, is one of the effective ways to treat nickel laterite. It has been successfully used in processing the iron-rich limonitic part of nickel laterite ore bodies. However, reserves of limonite ores are limited, and large deposits of saprolitic ores (with high Mg and Si content) cannot currently be economically processed by the Caron process.
Ore Preparation and Composition
A typical oxide ore treated by the Caron process contains various metal oxides as shown in Table 1 below.
Table 1. Typical composition of an oxide ore
| %Ni | %Co | %Fe | %Mg | %SiO₂ |
| 1.26 | 0.1 | 46.5 | 0.1 | 1.9 |
The preparation process begins with the ore, which typically contains 20-50% moisture after mining. The material is:
- Crushed to less than 1 inch in size
- Dried to reduce moisture content to 1-3%
This preparation increases the specific surface area, making the ore more responsive to roasting and leaching treatments.
Reduction Roasting Process
The dried ore enters the reduction roasting stage, where it is gradually heated to a specified temperature under a reducing atmosphere. Figure 1 illustrates the block diagram of the complete Caron process.
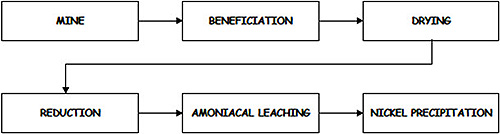
Figure 1: Block diagram for Caron process
The primary objective of roasting is to reduce nickel oxide to metallic nickel, making it amenable to ammoniacal leaching. A critical aspect is achieving selective reduction of nickel and cobalt while minimizing iron reduction.
The primary reactions during roasting affect nickel and iron as shown in equations (1) to (9):
NiO(s) + H2(g) = Ni(s) + H2O(g) (1)
3 Fe2O3(s) + H2(g) = 2 Fe3O4(s) + H2O(g) (2)
Fe3O4(s) + H2(g) = 3 FeO(s) + H2O(g) (3)
FeO(s) + H2(g) = Fe(s) + H2O(g) (4)
NiO(s) + CO(g) = Ni(s) + CO2(g) (5)
3 Fe2O3(s) + CO(g) = 2 Fe3O4(s) + CO2(g) (6)
Fe3O4(s) + CO(g) = 3 FeO(s) + CO2(g) (7)
FeO(s) + CO(g) = Fe(s) + CO2(g) (8)
Ni(s) + Fe(s) = FeNi(s) (9)
Similar reactions occur for cobalt and copper. The reduction of metal oxides primarily takes place at temperatures between 700-900°C. Temperature affects reaction kinetics, with higher temperatures producing faster reactions. Most operations maintain a practical lower limit of approximately 540°C and an upper limit of about 900°C to avoid sintering.
Composition After Reduction and Cooling Process
To obtain maximum reduction of nickel oxide to metallic nickel, most of the iron must also be reduced, as a substantial fraction of nickel in laterite ores is associated with iron oxides. However, complete reduction of iron to metallic form is unnecessary and undesirable.
After reduction, the material is cooled to 120-150°C under an inert atmosphere to prevent re-oxidation of nickel and other desired metals. Table 2 shows a typical composition of reduced oxide ore after losing approximately 20% of its original mass.
Table 2. Typical composition of the reduced oxide ore
| %Ni | %Co | %Fe | %Mg | %SiO₂ |
| 1.58 | 0.13 | 58.1 | 0.13 | 2.38 |
Ammoniacal Leaching and Recovery
The cooled, roasted ore proceeds to ammoniacal leaching, where metallic nickel is oxidized and dissolved. The efficiency of metal solubilization depends heavily on the reduction roast conditions, which vary according to ore type.
Equations (10) to (13) represent the typical overall leaching reactions. The iron-ammine complex remains stable only under limited conditions. In oxidizing environments, this complex decomposes to form insoluble hydroxide. Air is sparged into the leach tanks to provide the necessary oxygen.
Figures 2 below demonstrates the thermodynamic considerations in this process.
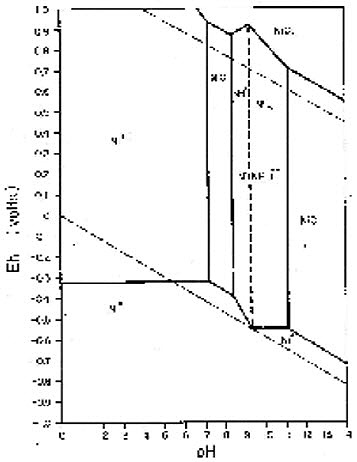
Figure 2: Eh-pH diagram for Ni-NH₃-H₂O system at 298 K and 1 atm total pressure
The diagrams show that at pH between 9 and 10 under oxidative conditions, Ni(NH₃)⁺⁺ is the thermodynamically predominant nickel species, while Fe(OH)₃(s) is the predominant iron species. This selective complexation allows for effective separation of nickel from iron during the leaching process.
Find Instantly Precise Compositions of Materials!
Total Materia Horizon contains chemical compositions of hundreds of thousands materials and substances, as well as their mechanical and physical properties and much more.

Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.