Spray Forming of Aluminum-Alloy Products: Part One
Abstract
Spray forming offers a combination of low-cost manufacturing with enhanced properties and performance. As such, it has emerged as a key competitor for existing technologies, such as casting, ingot metallurgy, electrode remelting processes, and powder metallurgy.
As well as potential near-net-shape benefits, the primary advantage of the spray forming process is the ability to manufacture alloy compositions that are problematical in conventional processes.
Spray forming offers a combination of low-cost manufacturing with enhanced properties and performance. As such, it has emerged as a key competitor for existing technologies, such as casting, ingot metallurgy, electrode remelting processes, and powder metallurgy.
The spray forming process is an advanced casting process for the manufacture of billet materials and is shown schematically in Figure 1. Spray forming comprises the sequential steps of:
- continuous gas atomization of a melt stream to produce a spray of 10- to 500-μm-diameter alloy droplets,
- droplet cooling at typically 102 to 104 K/s and acceleration to 50 to 100 m/s under the action of the atomizing gas,
- deposition of droplets at the growing spray-formed billet surface, and
- relatively slow cooling at 0.1 to 10 K/s and solidification of any residual liquid in the spray-formed billet.
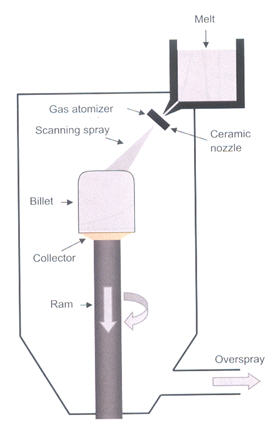
Figure 1: Schematic of the spray forming process for the manufacture of billets.
As well as potential near-net-shape benefits, the primary advantage of the spray forming process is the ability to manufacture alloy compositions that are problematical in conventional processes such as ingot casting, direct chill casting, and powder metallurgy. Commercial examples of alloys manufactured by spray forming include AI-Si based alloys for cylinder liners, high speed and speciality steel billets and Si-Al alloys for thermal management applications. The disadvantages of spray forming include as-sprayed porosity that requires closing by hot isostatic pressing or other downstream processes, and losses because not all droplets created by atomization end up in the spray- formed billet.
Despite many investigations of the relationship between the spray forming conditions, the solidification conditions arising, and the as-sprayed microstructure, some uncertainties remain in how the underlying process physics and mechanics give rise to the advantageous spray-formed microstructure that typically comprises the following:
- equiaxed/polygonal grains of diameter typically in the range 20 to 50 μm and the complete absence of columnar/dendritic morphologies,
- high levels of microstructural homogeneity and low levels of microsegregation regardless of position in the billet, and
- the ability to produce this characteristic spray-formed microstructure in all engineering alloy systems.
For example, Figure 2(a) shows a 26 kg AI-5.3Mg-1.2Li-0.28Zr alloy billet spray formed at Oxford University, and the corresponding as-spray-formed microstructure is shown in the electron backscattered diffraction (EBSD) orientation map in Figure 2(b). The as-spray-formed grain size was ≈15 μm and the porosity was <1 area pct. Because of the broad range of droplet sizes produced by atomization, there is always a wide range of droplet thermal conditions within the spray at deposition.

Figure 2: (a) A typical 26-kg AI-5Mg-1.2Li-0.28Zr billet spray formed at Oxford University and (b) an AI-5Mg-l.2Li-0.28Zr as-sprayed microstructural orientation map obtained by EBSD showing noncolumnar/dendritic equiaxed polygonal grains characteristic of the spray forming process.
A simple model has been described to relate the distribution of droplet diameters and solid fraction at deposition to the embryonic grain diameter in the billet top surface region. Calculated embryonic grain diameters of 11 to 58 μm are in good agreement with experimental measurements of final grain diameters in a range of alloys. Model predictions are relatively insensitive to assumed deposition conditions, because the embryonic grain diameter is determined primarily by the large number of fully solid particles always present in the spray. Although these particles contribute little in terms of volume to the final billet, they are critical in determining the distribution of embryonic grain diameters.
The large thermal mass associated with the larger liquid droplets must be sufficient to partially remelt the solid component of the spray on deposition so that the billet top surface is mushy. The reheating and remelting processes that must occur for a large number of depositing solid and mushy droplets destabilizes their dendritic/cellular microstructure, causing it to break up and providing a grain multiplication effect.
The resulting high number of small solid fragments in the mushy billet top surface and the corresponding small interdiffusion distances promote rapid thermal and compositional equilibration that helps to reduce microsegregation. All remnants of the droplet microstructure are removed and the solid component in the billet top surface region spheroidizes in the relatively flat temperature gradient to reduce solid/liquid interfacial area. In the subsequent relatively slow cooling of the still forming billet, the spheroidized solid coarsens quickly in the early stages due to small interdiffusion distances and highly mobile liquid films between embryonic grains.
Find Instantly Thousands of Metallography Diagrams!
Total Materia Horizon contains a unique collection of metallography images across a large range of metallic alloys, countries, standards and heat treatments.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.