Nickel Flash Smelting
Abstract
Nickel flash smelting represents an efficient industrial process for converting sulfide nickel concentrates into nickel matte and slag. This technology processes sulfide ores from major producers like Canada and Russia, which together account for 40-50% of global nickel production. The flash smelting furnace utilizes oxygen-enriched air and controlled partial oxidation to melt concentrate particles, creating immiscible phases that separate by density. The resulting matte undergoes conversion in Peirce-Smith converters to produce high-grade matte for refinery processing, while slags are recycled through electric furnaces to recover additional metal values. This integrated approach maximizes nickel recovery while maintaining process efficiency in commercial operations.
Introduction to Nickel Properties and Commercial Significance
Nickel (Ni) is a silvery-white, hard metal that forms compounds in several oxidation states. The divalent ion proves most important for both organic and inorganic substances, though trivalent forms may develop through redox reactions in cellular environments. Key nickel compounds with practical water insolubility include carbonate, sulfides (primarily amorphous or crystalline monosulfide NiS and subsulfide Ni₃S₂), and oxides (NiO, Ni₂O₃). These water-insoluble nickel compounds may dissolve in biological fluids, with particles of identical chemical composition exhibiting different biological activity depending on crystalline structure and surface properties.
Global Nickel Ore Classification and Production
Two commercial classes of nickel ore dominate global production: sulfide ores (pentlandite and pyrrhotite) and silicate-oxide ores. Most commercial nickel originates from sulfide ores, with Canada and the Russian Federation serving as the largest producers. These two nations each contribute 20-25% of total annual production, which reached 784,820 tons in 1988.
Flash Smelting Process Overview
Primary Processing in the Flash Smelting Furnace
Nickel flash smelting technology processes sulfide nickel concentrate using the Outotec® Flash Smelting Furnace to produce nickel matte and slag. The dried feed mixture combines with oxygen-enriched process air and distribution air in the concentrate burner, ensuring even distribution throughout the reaction shaft suspension.
As the dried charge flows downward through the reaction shaft, concentrate particles undergo dissociation, ignition, and controlled partial oxidation. This process generates substantial heat, causing material melting into fine droplets. Within the settler section, immiscible slag and matte phases separate into distinct layers based on their specific densities.
Process gases flow upward through the uptake shaft toward a heat recovery boiler for cooling. The boiler separates flue dust from the gas stream, while remaining fine dust undergoes recovery in an electrostatic precipitator. Dust collected from both the boiler and precipitator returns to the furnace for reprocessing.
Slag Processing and Electric Furnace Operations
The flash furnace produces slag that combines with converter slag for processing in an electric slag cleaning furnace before granulation and disposal. Reduction occurs batch-wise using coke as the reducing agent. During settling periods, reduced metal droplets and mechanically trapped matte droplets settle through the slag layer to the electric furnace bottom, forming the matte layer. Immiscible slag components with lower density remain at the bath surface, creating the cleaned slag layer.
Conversion Process in Peirce-Smith Converters
Mattes from both the flash smelting furnace and the electric Outotec® Slag Cleaning Furnace undergo conversion in Peirce-Smith converters using oxygen-enriched air blown through tuyeres. Silica flux addition facilitates slag formation during this process. The resulting high-grade matte undergoes granulation for further refinery treatment, while waste slag receives granulation before transfer to disposal areas.
Flash Smelting Furnace Design and Operation
Furnace Structure and Components
A typical flash smelting furnace features a refractory-lined vessel comprising three main sections: the reaction shaft, settler, and uptake shaft. The concentrate burner, positioned at the reaction shaft top, plays a vital role in furnace operation through its optimal design configuration.
The concentrate burner incorporates several concentric ducts that facilitate the blowing and mixing of process gas and concentrate within the furnace. This burner's primary function involves creating an optimal suspension of solid particles and oxygen-enriched process air throughout the reaction shaft.
Combustion Process and Heat Recovery
Individual particles experience heating as they circulate within the furnace atmosphere. Following ignition, particles begin combusting with oxygen present in the process gas. Combustion reactions involving fine sulfides (particles <100μm) proceed rapidly, releasing substantial heat quantities that achieve complete melting of combusting particles and other feed mixture materials.
Molten particles flow downstream for collection in the settler, where silicate slag and sulfidic matte layers undergo separation. Off-gas composition, primarily an SO₂-N₂ mixture, flows through the uptake to the waste heat boiler for cooling and heat content recovery as steam.
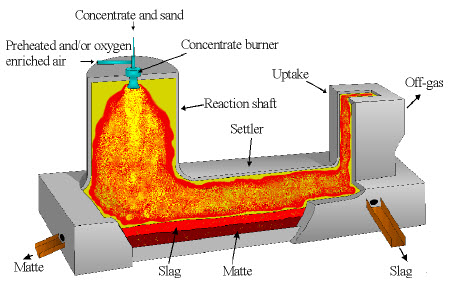
Figure 1: Flash smelting furnace with its main sections and material flows
Access Precise Properties of Nickel and Superalloys Now!
Total Materia Horizon contains property information for 20,000+ nickel alloys: composition, mechanical and physical properties on various temperatures, corrosion resistance, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.