Microstructures in Austenitic Stainless Steels
Abstract
Austenitic stainless steels undergo significant microstructural changes during exposure to elevated temperatures, with both short-term and long-term transformations affecting their properties. Initially, M23C6 carbides form during brief heat exposure, while extended aging leads to intermetallic phase precipitation and carbide dissolution. These transformations substantially influence both mechanical performance and corrosion resistance. This article examines the microstructural evolution in austenitic stainless steels during welding and high-temperature exposure, with particular focus on secondary phase formation, characterization techniques, and the relationship between microstructural stability and material properties in various industrial applications.
Introduction to Austenitic Stainless Steels
Stainless steels represent a specialized group of high-alloy steels based primarily on the Fe-Cr, Fe-Cr-Ni, and Fe-Cr-C systems. Among these, austenitic stainless steels constitute a particularly important category widely utilized across numerous industries and environments. The standard austenitic composition typically features approximately 18% chromium and 8% nickel.
While stainless steels are generally considered weldable materials, proper fabrication requires adherence to specific guidelines to ensure defect-free construction and expected service performance. Welding processes invariably cause significant microstructural changes in both the weld metal and heat-affected zone compared to the base metal. The susceptibility to weld solidification and liquation cracking depends primarily on the composition of both base and filler metals, with particular sensitivity to impurity elements such as sulfur and phosphorus.
High-Temperature Microstructural Transformations
Austenitic steels experience distinctive microstructural changes during exposure to elevated temperatures, with transformation patterns differing between short-term and long-term exposure. Research by Heino et al. demonstrated that during heat treatment between 600-900°C, short-duration precipitation from austenite (less than 60 seconds) typically results in the formation of M23C6 carbide. When aging periods extend longer, additional precipitates including intermetallic phases form, generally accompanied by the dissolution of existing carbides.
These intermetallic precipitations hold particular significance not only for their considerable influence on mechanical properties but also for their pronounced effect on corrosion resistance. Numerous studies have investigated the microstructural behavior and precipitation patterns of stainless steels during welding and exposure to high temperatures.
Microstructure Stability in Austenitic Stainless Steels
Microstructural stability represents one of the most critical requirements for establishing appropriate mechanical and corrosion properties in Austenitic Stainless Steels (ASSs). To achieve stable microstructures, these steels typically undergo solution heat treatment followed by annealing at temperatures between 700–1100 K. During the annealing process, secondary phases precipitate from the austenite (matrix phase with a face-centered cubic crystal lattice) and/or the δ-ferrite (high-temperature phase with a body-centered cubic crystal lattice).
Table 1. The secondary phases in austenitic stainless steels (Note: concentrations of non-metallic elements are not included in this table)
| Phase/Crystal Lattice | Lattice Parameter (nm) | Fe (%) | Cr (%) | Ni (%) | Mo (%) | Si (%) | Steel |
| σ-phase, tetragonal | a = 0.883 | 49-52 | 33-34 | 4-7 | 1 | 8-11 | |
| AISI 316 | |||||||
| a = 0.461 | 52 | 38 | 5 | 10 | 1 | 19Cr-12Ni-0.02C | |
| a = 0.883 | 52 | 38 | 4-7 | 10 | 1 | AISI 316 | |
| 19 | 44-48 | 2-6 | 4-12 | - | 20Cr-25Ni-4Mo | ||
| Laves, hexagonal | a = 0.475 | 37 | 11 | 1 | - | - | AISI 316 |
| a = 0.4773 | 35 | 11 | 1 | - | - | AISI 316 | |
| a = 0.473 | 38 | 4 | 3 | 43-47 | - | 316-LN-3 | |
| χ-phase, cubic | a = 0.890 | 51-53 | 23-24 | 3-4 | 18 | 1 | AISI 316 |
| a = 0.890 | 51 | 24 | 4 | 18 | 1 | 316-LN-3 | |
| 51 | 23-24 | 4 | 18 | 1 | UNS S31803 | ||
| 51-53 | 24 | 4 | 18 | 1 | AISI 316A30X | ||
| M23C6, cubic | a = 1.0905 | 14 | 17 | 1 | 4 | - | 19Cr-12Ni-25Si-0.02C |
| 16-23 | 18 | 1 | 4 | - | 20Cr-25Ni-4Mo | ||
| MX, cubic | a = 0.422 | - | - | - | - | - | Ti = 99, AISI 321 |
| Cr2N, hexagonal | a = 0.4776 | 100 | - | - | - | - | 316-LN-3 |
| Z-phase, tetragonal | a = 1.00 | - | 23-24 | 3-4 | 7 | 1 | 20Cr-25Ni-4Mo |
| R-phase, cubic | a = 1.093 | ||||||
| 55-58 | 6-7 | - | 20 | 1 | UNS S31803 | ||
| R-phase, hexagonal | a = 1.934 | 23-27 | 5 | - | 25 | - | 20Cr-25Ni-4Mo |
In ASSs containing higher bulk carbon content (typically 0.3-0.6% by mass), the orthorhombic M7C3 carbide may also develop. Specialized ASSs with elevated silicon or phosphorus content may additionally form silicides (G-phase with a body-centered cubic crystal lattice) and phosphides (M3P with a tetragonal crystal lattice). Some industrial applications utilize ASSs in their as-cast condition, such as centrifugally cast or sand-cast large tubes and elbows for pipeline systems. Given the highly heterogeneous microstructure of as-cast steels, understanding the proportion, chemical composition, and stability of phases (particularly δ-ferrite) becomes essential.
Characterization Techniques for Phase Identification
Information about phases present in ASSs can be obtained through both experimental and theoretical approaches. Modern phase identification employs precise experimental techniques including transmission electron microscopy (TEM) with energy-dispersive X-ray spectroscopy (EDX), electron energy loss spectroscopy (EELS), and electron diffraction. Additional techniques include Scanning Electron Microscopy with Energy Dispersive X-Ray Analysis (SEM–EDX), Wavelength Dispersive X-ray diffraction (WDX), and Differential Thermal Analysis (DTA). These experimental methods are increasingly combined with thermodynamic predictions of phase equilibrium and modeling of phase evolution.
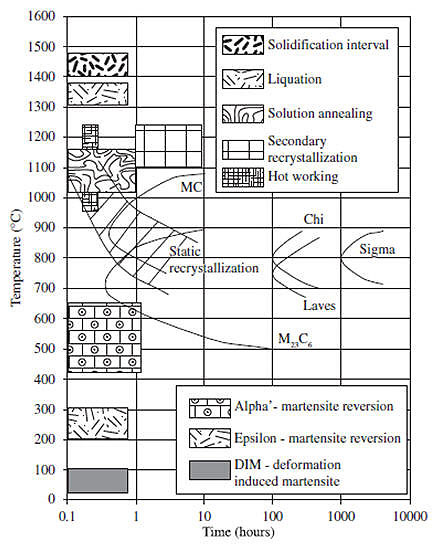
Figure 1: Main transformations that occur in austenitic stainless steels between room temperature and the liquid state.
Conclusion
The microstructural evolution of austenitic stainless steels during heat treatment and service conditions significantly affects their performance characteristics. Understanding these transformations, particularly the formation of secondary phases, provides critical insights for controlling material properties in various applications. The combination of advanced characterization techniques with theoretical models offers comprehensive tools for predicting and managing microstructural changes in these important engineering materials.
Find Instantly Thousands of Metallography Diagrams!
Total Materia Horizon contains a unique collection of metallography images across a large range of metallic alloys, countries, standards and heat treatments.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.