Hardenable Alloy Steels
Abstract
By far the largest tonnage of alloy steels is of the types containing generally 0.25 to 0.55% C, or less, and usually quenched and tempered for high strength and toughness. Manganese, silicon, nickel, chromium, molybdenum, vanadium, aluminum and boron are commonly present in these steels to enhance the properties obtainable after quenching and tempering.
By far the largest tonnage of alloy steels is of the types containing generally 0.25 to 0.55% C, or less, and usually quenched and tempered for high strength and toughness. Manganese, silicon, nickel, chromium, molybdenum, vanadium, aluminum and boron are commonly present in these steels to enhance the properties obtainable after quenching and tempering.
These alloy steels are ordinarily quench-hardened and tempered to the level of strength desired for the application. Even though the strength level at which the steel is used may be as low as, or lower than, that which could be achieved by the microstructure (fine pearlite or upper bainite) developed by a simple cooling from forging or normalizing temperature, the steels are quench-hardened and tempered, indirectly reflecting the engineering and economic basis of the demand for this type of steel.
The microstructure (tempered martensite or bainite) produced by quenching and tempering these alloy steels is characterized by a greater toughness or capacity to deform without rupture at any strength level. Similarly, under the adverse state of stress below a notch in bending, the tempered martensite may flow considerably at a testing temperature far below that at which a pearlitic steel of equal strength would break in a brittle manner; the Charpy or Izod values are thus improved. The basic phenomenon of developing this favorable microstructure by heat treatment is manifested in plain carbon steels, but only in small sections; thus the most important effect of the alloying elements in these steels is to permit the attainment of this microstructure, and the accompanying superior toughness in larger sections.
Alloying Elements Dissolved in Austenite. The general effect of elements dissolved in austenite is to decrease the transformation rates of the austenite at subcritical temperatures. The only one among the common alloying elements to behave exceptionally in this regard is cobalt. Since the desirable products of transformation in these steels are martensite and lower bainite, formed at low temperatures, this decreased transformation rate is essential; it means that pieces can be cooled more slowly, or larger pieces can be quenched in a given medium, without transformation of austenite to the undesirable high-temperature products, pearlite or upper bainite.
This function of decreasing the rates of transformation, and thereby facilitating hardening to martensite or lower bainite, is known as hardenability and is the most important effect of the alloying elements in these steels. Thus, by increasing hardenability the alloying elements greatly extend the scope of enhanced properties in hardened and tempered steel to the larger sections involved in many applications.
The several elements commonly dissolved in austenite prior to quenching, increase hardenability in approximately the following ascending order: nickel, silicon, manganese, chromium, molybdenum, vanadium and boron. The effect of aluminum on hardenability has not been accurately evaluated. But at 1% Al, as used in "nitralloy" steels, the effect on hardenability seems to be relatively small. Further, it has been found that the addition of several alloying elements in small amounts is more effective in increasing hardenability than the addition of much larger amounts of one or two.
In order to increase hardenability effectively, it is essential that the alloying elements are dissolved in austenite. The steels containing the carbide-forming elements - chromium, molybdenum and vanadium - require special consideration in this respect. These elements are present predominantly in the carbide phase of annealed steels, and such carbides dissolve only at higher temperatures and more slowly than iron carbide. Since the basic function of the alloying elements in these steels is to increase hardenability, the selection of steel and the choice of suitable austenitizing conditions should be based primarily on the assurance of adequate hardenability. More than adequate hardenability is rarely a disadvantage, except in cost.
Alloying Elements in Quenching. Since the sections treated are often relatively large, and since the alloying elements have the general effect of lowering the temperature range at which martensite is formed, the thermal and transformational stresses set up during quenching tend to be greater in these alloy steel parts than those involved in quenching the necessarily smaller sections of plain carbon steels. In general, this means greater distortion and risk of cracking.
The alloying elements, however, have two functions that tend to offset these disadvantages. The first and probably the most important of these functions is that of permitting the use of lower carbon content for a given application. The decrease in hardenability accompanying the decrease in carbon content may be offset very readily by the hardenability effect of the added alloying elements, and the lower-carbon steel will exhibit a much lower susceptibility to quench cracking. This lower susceptibility results from the greater plasticity of the low-carbon martensite and from the generally higher temperature range at which martensite is formed in the lower-carbon materials. Quench cracking is seldom encountered in steels containing 0.25% C or less, and the susceptibility to cracking increases progressively with increasing carbon content.
The second function of the alloying elements in quenching is to permit slower rates of cooling for a given section, because of increased hardenability, and thereby generally to decrease the thermal gradient and, in turn, the cooling stress. It should be noted, however, that this is not altogether advantageous, since the direction, as well as the magnitude, of the stress existing after the quench, is important in relation to cracking.
In order to prevent cracking, the surface stresses after quenching should be either compressive or at a relatively low tensile level and, under certain circumstances, lowering the cooling rate may lead to increased tensile stresses at the surface, thus increasing the tendency to crack. In general, though, unless a study of the particular piece being quenched indicates that it falls within this category of increased susceptibility with decreased quenching rates, the use of a less drastic quench suited to the hardenability of the steel will result in lower distortion and greater freedom from cracking.
Furthermore, the increased hardenability of these alloy steels may permit heat treatment by "austempering" or "martempering", and thereby the level of adverse residual stress before tempering may be held to a minimum. In "austempering", the work piece is cooled rapidly to a temperature in the lower bainite region and is allowed thereafter to transform, completely at some chosen temperature. Since this transformation occurs at a relatively high temperature and proceeds rather slowly, the stress level after transformation is quite low and distortion is held to a minimum.
In "martempering", the piece:
- is cooled rapidly at the surface to a temperature that permits very little martensite to form, if any;
- is equalized at this temperature; and
- is then cooled slowly so that transformation throughout the whole section occurs more or less simultaneously, thereby holding transformational stresses at a very low level and minimizing distortion and danger of cracking.
Alloying Elements in Tempering. Hardened steels are softened by reheating, but this effect is not the one actually sought in tempering. The real need for increasing the capacity of the piece is to flow moderately without fracture, and this is inevitably accompanied by a loss of strength. Since the tensile strength is very closely related to hardness in this class of steels, as heat-treated, it is satisfactory to follow the effects of tempering by measuring the Brinell or Rockwell hardness. The statistical mean of the relationships among Brinell hardness, tensile strength and yield strength, is shown in Fig. 1, drawn from many data.
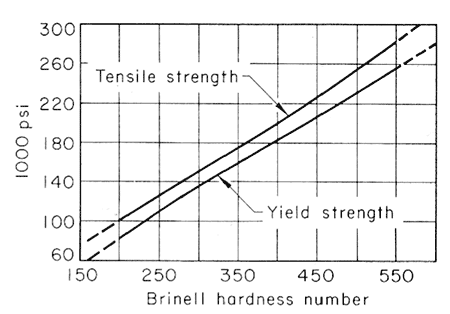
Figure 1.
Figure 2-4 shows the softening pattern of nine 0.45% C steels with increase in the tempering temperature, for one hour. Somewhat shorter or longer intervals at temperature would show little difference in hardness values.
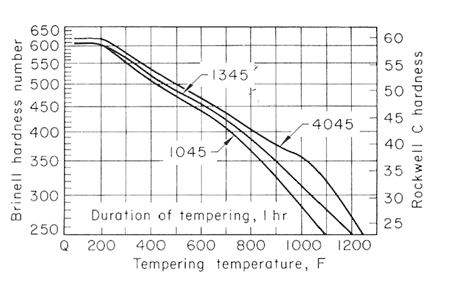
Figure 2.
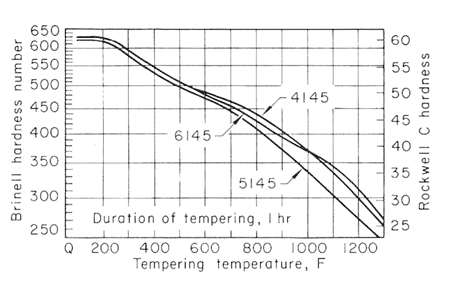
Figure 3.
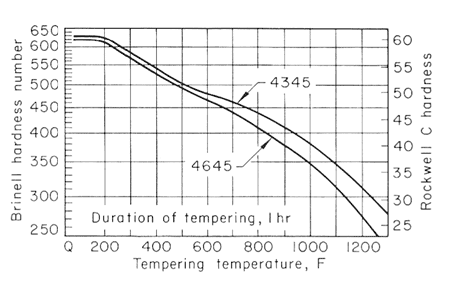
Figure 4.
As a first approximation, the softening pattern of steels similar but differing in carbon content through the range from 0.25 to 0. 55% C may be estimated from Fig. 3.
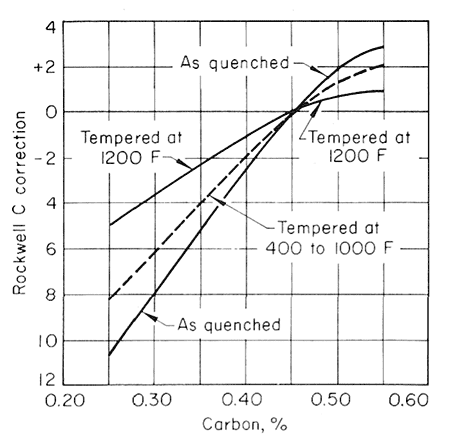
Figure 5.
This illustrates the general effect of carbon content on this softening pattern in terms of Rockwell C hardness units to be added to or subtracted from the 0.45% C value at different levels of tempering temperature, but accurate data that would permit a strictly quantitative relationship of this type are not available.
The effect of carbon content on the hardness of the tempered steels is much greater for the lower tempering temperatures than for 1200oF (649oC) and higher, and that the effect likewise decreases when there is more than 0.50% C. Figures 2,3,4 and 5, used together, make it possible to estimate the strength from a given tempering treatment applied to a given type of steel.
The general effect of the alloying elements is to retard the softening rate, so these alloy steels will require a higher tempering temperature to obtain a given hardness than carbon steel of the same carbon content. However, the individual elements show significant differences in the magnitude of their retarding effect. Nickel, silicon, aluminum and, to a large extent, manganese, which have little or no tendency to occur in the carbide phase, and merely remain dissolved in ferrite, have only a minor effect on the hardness of the tempered steel - an effect that would be expected from the general pattern of solid-solution hardening.
Chromium, molybdenum and vanadium, on the other hand, which migrate to the carbide phase when diffusion is possible, bring about a retardation of softening, particularly at the higher tempering temperatures. These elements do not merely raise the tempering temperature; when they are present in higher percentages, the rate of softening is no longer a continuous function of the tempering temperature. That is, the softening curves for these steels will show a range of tempering temperature in which the softening is retarded or, with relatively high alloy content, in which the hardness may actually increase with increasing tempering temperature. This characteristic behavior of the alloy steels containing the carbide-forming elements is known as "secondary hardening" and results presumably from a delayed precipitation of fine alloy carbides.
Find Instantly Thousands of Heat Treatment Diagrams!
Total Materia Horizon contains heat treatment details for hundreds of thousands of materials, hardenability diagrams, hardness tempering, TTT and CCT diagrams, and much more.

Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.