Heat-Resisting Alloys
Abstract
Heat-resisting alloys useful at temperatures above 1200oF are based on iron, on nickel and on cobalt and contain elements that form precipitates that harden the matrix after solution treating and aging. Structural stability and resistance to oxidation and corrosion at elevated temperatures are required of these alloys.
Heat-resisting alloys useful at temperatures above 1200oF are based on iron, on nickel and on cobalt and contain elements that form precipitates that harden the matrix after solution treating and aging. Structural stability and resistance to oxidation and corrosion at elevated temperatures are required of these alloys.
Iron-base (actually, iron-chromium-nickel-base) alloys are the least costly and are applied in the lower temperature range, 1200 to 1500oF. Nickel-base and cobalt-base alloys are both applicable within the range of 1500 to 2000oF, and at temperatures below 1500oF as well. The hardening phase in nickel-base alloys is a nickel-aluminum-titanium phase called gamma prime. The hardening phase in cobalt-base alloys is complex carbide.
Vacuum melting permits accurate adjustment of composition and deoxidation with carbon, thus permitting oxygen removal in gaseous combination with carbon and inhibiting the formation of solid oxides in the bath. Under vacuum, gaseous hydrogen and nitrogen are removed to trace residuals. Vacuum melting also removes volatile metals, such as lead and zinc. Final additions of reactive metals are facilitated by the absence of any reaction of the bath with either air or slag.
For the most complex alloy systems, powder metallurgy is employed to prevent gross segregation. The alloy is melted in a conventional way and atomized while still in the liquid state, to form spheres, which are ground to fine powders of homogeneous chemical composition. The powders are compacted into preforms, sintered and then forged in the conventional way to produce segregation-free forgings.
A great many cast and wrought heat-resisting alloys are available. Iron-base heat-resisting alloys are only slightly more alloyed than stainless steels. They maintain useful strength within the lower range of temperatures, up to 1200oF; some are used at up to 1500oF. Figures 1 show rupture strengths for about 40 different compositions as a function of temperature. Ascoloy, with the curve shown at the extreme left in Fig. 1 over a temperature range of 900 to 1200oF, is a martensitic chromium stainless steel.
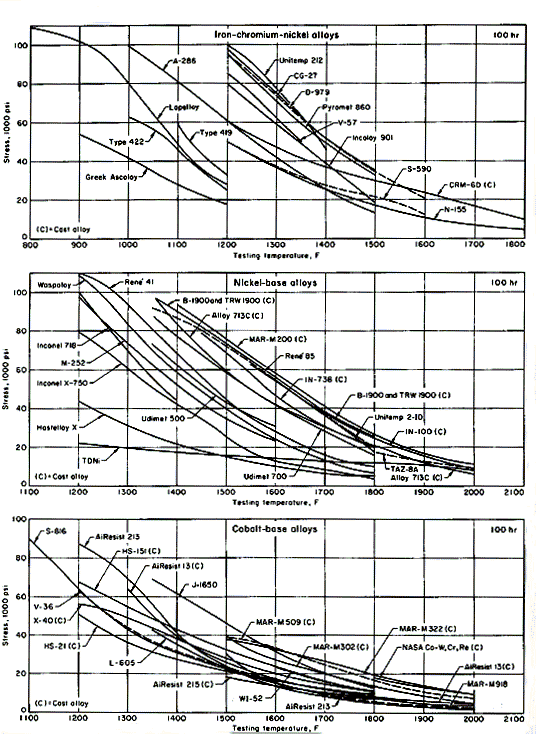
The curves shown on diagrams are typical and reflect neither statistical distribution nor specified minimums. Variations in composition, melting, forging and heat treatment are not reflected by these smoothed, typical curves. The curves therefore provide only a first approximation for material selection. Creep characteristics, microstructural stability, and resistance to corrosion by sulfur-containing gas at high temperature must also be taken into consideration.
Although developed originally for use at high temperature, some heat-resisting alloys have also been used at cryogenic temperatures, as forged components for handling liquid oxygen and liquid hydrogen.
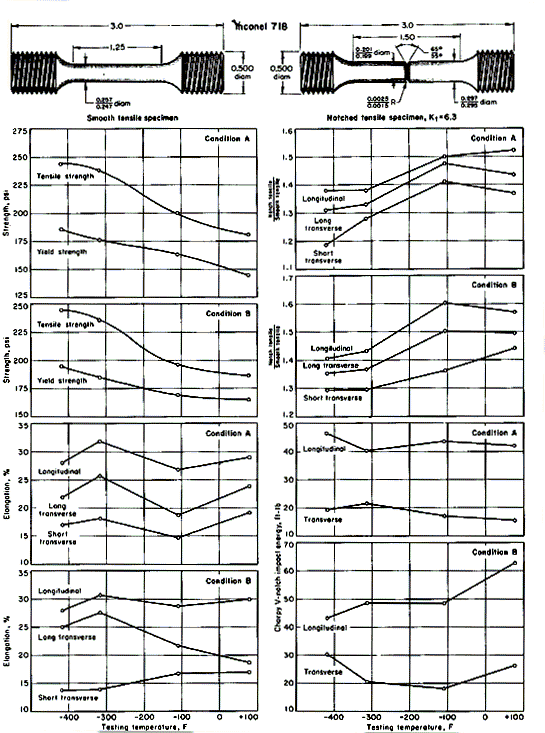
Mechanical-test results for Inconel 718 at room and cryogenic temperatures are shown in Fig. 2 for specimens cut from forged components of over-all dimensions 4x9x15 in. The forgings were produced from 6-in.-diameter billets broken down from an 18-in.-diameter ingot.
Test results shown in Fig. 2 include tensile, notch-tensile and Charpy impact values. Each plotted point is an average of four tests. Testing was at room temperature, at -110oF in gaseous nitrogen, at -320oF in liquid nitrogen, and at -423oF in liquid hydrogen. The test values that concern ductility (elongation, notch-tensile / smooth-tensile ratio, and impact toughness) are shown for both longitudinal and transverse directions. Longitudinal bars were machined parallel to the 15-in. dimension of the forging; long-transverse direction bars were machined parallel to the 9-in. dimension; and the short-transverse direction bars, parallel to the 4-in. dimension.
Find Instantly Precise Material Properties!
Total Materia Horizon contains mechanical and physical properties for hundreds of thousands of materials, for different temperatures, conditions and heat treatments, and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.