Steel Bainite Transformation
Abstract
Bainite is a microstructural constituent in steel consisting of ferrite and carbide phases that forms through controlled isothermal heat treatment of austenite. Discovered in the 1920s-1930s through pioneering work by Hultgren, Robertson, Davenport, and Bain, this structure exhibits exceptional mechanical properties combining high strength with excellent formability and toughness. The bainite transformation occurs when austenite is cooled below the eutectoid temperature and held isothermally between 200°C and 600°C. The transformation start temperature (Bs) depends significantly on carbon content and alloying elements, with empirical equations available for prediction. Bainitic microstructures range from upper bainite with lath-shaped ferrite to lower temperature variants with plate-like morphology, making bainite steels valuable for high-strength applications across various industries.
Introduction to Bainite Steel Transformation
Bainite represents one of the most important microstructural constituents in modern steel metallurgy. As a mixture of ferrite and carbide phases—typically cementite—decomposed from austenite, bainite steels offer an exceptional combination of mechanical properties that make them indispensable in engineering applications. The discovery and development of bainite transformation processes have revolutionized steel heat treatment, providing metallurgists with powerful tools to optimize strength, formability, and toughness simultaneously.
Historical Development of Bainite Discovery
The discovery of bainite in steels emerged directly from the invention of isothermal heat treatment techniques, which catalyzed numerous breakthroughs in austenite decomposition research during the 1920s and 1930s. This period marked a golden age of metallurgical discovery that fundamentally changed our understanding of steel microstructures.
Hultgren pioneered this field in 1920 by utilizing isothermal heat treatment to identify what he termed "secondary ferrite" within a martensitic matrix. Building upon this foundation, Robertson published comprehensive metallographic research in 1929 that demonstrated how austenite decomposition progresses at various temperatures during isothermal treatment. Although Robertson's work provided crucial insights into temperature effects, it did not address the critical time dependence of these transformations.
The breakthrough came in 1930 when Davenport and Bain revolutionized the isothermal technique by implementing efficient quenching methods using thin specimens. Their innovative approach enabled them to capture and analyze partially transformed specimens, publishing detailed micrographs that revealed the true nature of this new microstructure. Their methodological advances directly led to the development of time-temperature transformation (TTT) diagrams, which remain among the most valuable tools in contemporary steel research.
When Davenport and Bain first observed bainite units, they described the structure as an "acicular, dark etching aggregate" that shared characteristics with both pearlite and martensite in similar steel compositions. Initially, they designated this structure "martensite-troostite" because its etching behavior differed distinctly from both martensite and troostite (fine pearlite). Subsequent research revealed that this structure exhibited superior toughness compared to tempered martensite of equivalent hardness—a discovery that immediately sparked extensive research interest due to its promising mechanical properties.
In recognition of Bain's fundamental contributions to the field, his colleagues proposed the name "bainite" in 1934. After several years of evaluation and acceptance within the metallurgical community, the term "bainite" became universally adopted and remains the standard nomenclature today.
Bainite Formation Process and Heat Treatment
Bainite formation occurs through a carefully controlled heat treatment process that transforms austenite into this beneficial microstructure. The process begins by heating steels or cast irons above their eutectoid reaction temperature, typically around 727°C for plain carbon steels, to achieve complete austenitization. This austenitizing process ensures uniform austenite formation throughout the material.
The critical transformation occurs when austenite cools below the eutectoid temperature and is subsequently held at an isothermal temperature below the pearlite transformation range. This isothermal holding temperature typically ranges between 200°C and 600°C, depending on the desired bainite characteristics and steel composition. Within this temperature window, austenite undergoes decomposition to form the characteristic bainite microstructure.
The transformation kinetics and final microstructure depend heavily on the specific isothermal temperature selected. Higher temperatures within the bainite range promote upper bainite formation with lath-shaped ferrite morphology, while lower temperatures favor different bainitic structures with varying mechanical properties.
Mechanical Properties and Engineering Applications
Bainite steel structures provide exceptional value in engineering applications due to their unique combination of mechanical properties. The primary advantage lies in achieving relatively high strength while maintaining excellent formability and toughness—characteristics that are often mutually exclusive in other steel microstructures.
Many high-strength steel grades incorporate bainite with varying carbon contents to optimize performance for specific applications. The versatility of bainite transformation allows metallurgists to tailor mechanical properties by adjusting carbon content, alloying elements, and heat treatment parameters. This flexibility makes bainite steels particularly valuable in automotive, construction, and energy industries where demanding mechanical property requirements must be met.
Bainite Transformation Start Temperature Calculations
Understanding and predicting the bainite transformation start temperature (Bs) is crucial for designing effective heat treatment processes. The Bs temperature varies significantly with carbon content and alloying element additions, necessitating accurate prediction methods for industrial applications.
Empirical Equations for Bs Temperature Prediction
Several empirical equations have been developed to predict Bs temperatures based on steel composition. Steven and Hayes established a widely-used equation for Bs as a function of composition (in wt.%) specifically for hardenable low-alloy steels containing 0.1-0.55% carbon:
Bs(°CC)= 830 - 270(%C) - 90(%Mn) - 37(%Ni) – (%70Cr) – 83(%Mo)
For specialized applications, Bodnar et al. developed an alternative equation tailored for low-carbon bainitic steels containing 0.15-0.29% carbon, particularly for high-temperature applications in the electric power industry:
Bs(°CC)= 844 - 597(%C) - 63(%Mn) - 16(%Ni) – (%78Cr)
These equations demonstrate the significant influence of carbon content and various alloying elements on bainite transformation kinetics, providing essential tools for heat treatment design and optimization.
Upper Bainite Microstructural Characteristics
The microstructural features of bainite exhibit remarkable similarities to martensite, particularly in terms of crystallographic relationships and morphological characteristics. The shape of bainitic ferrite (BF) undergoes a systematic transformation from lath-shaped to plate-like morphology as the transformation temperature decreases between the Bs temperature and the martensite start temperature (Ms).
Upper bainite (UB) specifically consists of lath-shaped ferrite and forms at higher temperatures within the bainite transformation range.
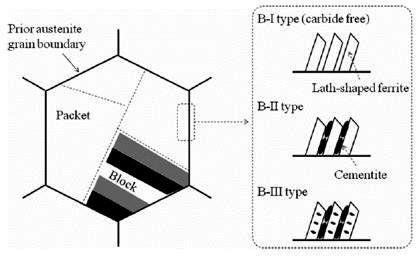
Figure 1: The schematic image of the morphological features of the upper bainite structure
Figure 1 provides a schematic representation of the crystallographic and morphological features characteristic of upper bainite structures.
The crystallographic relationship between the prior austenite and bainitic ferrite closely approximates the Kurjumov-Sachs orientation relationship, indicating a systematic transformation mechanism. During upper bainite formation, the prior austenite grain subdivides into distinct packets containing parallel elongated ferrite laths. These packets further subdivide into blocks consisting of bainitic ferrite laths with nearly identical crystallographic orientations.
The morphological and crystallographic similarities between upper bainite and lath martensite strongly suggest comparable transformation mechanisms, despite the different temperature ranges and kinetics involved. This relationship provides valuable insights into the fundamental nature of diffusional versus displacive transformations in steel microstructures.
Conclusion
Bainite transformation represents a cornerstone achievement in steel metallurgy, providing engineers with versatile microstructures that combine high strength with excellent formability and toughness. From its discovery in the early 20th century through modern applications, bainite continues to play a crucial role in advanced steel development. Understanding bainite formation kinetics, transformation temperatures, and microstructural characteristics enables optimization of heat treatment processes for diverse engineering applications, ensuring continued relevance in evolving industrial demands.
Find Instantly Thousands of Heat Treatment Diagrams!
Total Materia Horizon contains heat treatment details for hundreds of thousands of materials, hardenability diagrams, hardness tempering, TTT and CCT diagrams, and much more.

Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.