High Temperature Corrosion: Part One
Abstract
High-temperature corrosion is a form of corrosion that does not require the presence of a liquid electrolyte.
Strictly speaking, high-temperature oxidation is only one type of high-temperature corrosion, but it is the most important high-temperature corrosion reaction.
High-temperature corrosion is a form of corrosion that does not require the presence of a liquid electrolyte. Sometimes, this type of damage is called dry corrosion or scaling. The term oxidation is ambivalent because it can either refer to the formation of oxides or to the mechanism of oxidation of a metal (i.e., its change to a higher valence than the metallic state).
Strictly speaking, high-temperature oxidation is only one type of high-temperature corrosion, but it is the most important high-temperature corrosion reaction. In most industrial environments, oxidation often participates in the high-temperature corrosion reactions, regardless of the predominant mode of corrosion.
Alloys often rely upon the oxidation reaction to develop a protective scale to resist corrosion attack such as sulfidation, carburization, and other forms of high temperature attack. In general, the names of the corrosion mechanisms are determined by the most abundant dominant corrosion products. For example, oxidation implies oxides, sulfidation implies sulfides, sulfidation/oxidation implies sulfides plus oxides, and carburization implies carbides.
Oxidizing environments refer to high-oxygen activities, with excess oxygen. Reducing environments are characterized by low-oxygen activities, with no excess oxygen available. Clearly, oxide scale formation is more limited under such reducing conditions. It is for this reason that reducing industrial environments are generally considered to be more corrosive than the oxidizing variety.
However, there are important exceptions to this generalization. At high temperatures, metals can react “directly” with the gaseous atmosphere. Electrochemical reaction sequences remain, and act as the underlying mechanism of high-temperature corrosion. The properties of high-temperature oxide films, such as their thermodynamic stability, ionic defect structure, and detailed morphology, play a crucial role in determining the oxidation resistance of a metal or alloy in a specific environment. High-temperature corrosion is a widespread problem in various industries such as
• Power generation (nuclear and fossil fuel) • Aerospace and gas turbine • Heat treating • Mineral and metallurgical processing • Chemical processing • Refining and petrochemical • Automotive • Pulp and paper • Waste incineration
Thermodynamic Principles: Standard free energy of formation versus temperature diagrams
Often determination of the conditions under which a given corrosion product is likely to form is required (i.e., in selective oxidation of alloys). The plots of the standard free energy of the reaction (ΔG0) as a function of temperature, commonly called Ellingham diagrams, can help to visualize the relative stability of metals and their oxidized products.
Figure 1 shows an Ellingham diagram for many simple oxides. The values of ΔG0 on an Ellingham diagram are expressed as kilojoules per mole of O2 to normalize the scale and be able to compare the stability of these oxides directly (i.e., the lower the position of the line on the diagram, the more stable is the oxide).
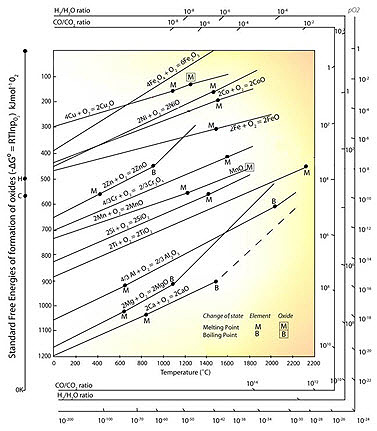
Figure 1: The Ellingham diagram for metallurgically important oxides.
For a given reaction [Equation (1)] and assuming that the activities of M and MO2 are taken as unity, Equation (2) or its logarithmic form [Equation (3)] may be used to express the oxygen partial pressure at which the metal and oxide coexist (i.e., the dissociation pressure of the oxide).
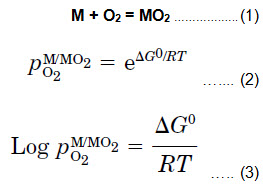
The values of 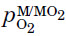 may be obtained directly from the Ellingham diagram by drawing a straight line from the origin marked O through the free-energy line at the temperature of interest and reading the oxygen pressure from its intersection with the scale at the right side labeled Log(pO2).
may be obtained directly from the Ellingham diagram by drawing a straight line from the origin marked O through the free-energy line at the temperature of interest and reading the oxygen pressure from its intersection with the scale at the right side labeled Log(pO2).
Values for the pressure ratio H2/H2O for equilibrium between a given metal and oxide may be obtained by drawing a similar line from the point marked H to the scale labeled H2/H2O ratio, and values for the equilibrium CO/CO2 ratio may be obtained by drawing a line from point C to the scale CO/CO2 ratio.
Read more
Access Precise Corrosion Properties Now!
Total Materia Horizon contains corrosion behaviour and property information for hundreds of thousands of materials, accross more than 2,000 media.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.