Epoxy Coated Reinforcement
Abstract
The corrosion of steel reinforcement is the primary and most costly form of deterioration currently impacting the performance of reinforced concrete bridge structures.
In the 1970s and 1980s, it was a commonly accepted view that epoxy coatings would provide superior corrosion protection for reinforcing steel in concrete. For that reason, epoxy coated reinforcement was routinely used in bridge decks and substructures. In addition to epoxy coated reinforcement, cathodic protection systems have also been used to protect bridge substructures.
BACKGROUND
Reinforced concrete is a durable material that will normally provide many years of relatively maintenance free life. However, experience shows that in hostile environments even good quality concrete will not necessarily protect reinforcing steel from corrosion for the long design life (typically 75 to 120 years) currently specified for many reinforced concrete structures.
Concrete is a naturally protective environment for steel reinforcement, its alkalinity (typically pH 12.5 to 13) allows steel to form a passive surface film, so for practical purposes the steel does not corrode. Protection from aggressive environments is provided by the surface concrete that covers the reinforcement and therefore the structural durability is a function of the concrete quality, and the depth of cover. If sufficient levels of chlorides reach the reinforcement or atmospheric carbon dioxide carbonates the cover, the natural protection is lost, the steel corrodes and expansion of the products of corrosion damages the surface concrete. Whilst this can become a serious structural problem it is normally only an inconvenient and costly maintenance issue, yet it is serious enough to justify the use of additional protection measures for new structures in hostile environments.
The corrosion of steel reinforcement is the primary and most costly form of deterioration currently impacting the performance of reinforced concrete bridge structures. In the United States, maintenance and replacement costs for deficient bridges are measured in billions of dollars.
In the 1970s and 1980s, it was a commonly accepted view that epoxy coatings would provide superior corrosion protection for reinforcing steel in concrete. For that reason, epoxy coated reinforcement was routinely used in North American bridge decks and substructures. In addition to epoxy coated reinforcement, cathodic protection systems have also been used to protect bridge substructures.
Eliminating or slowing the deterioration of reinforced concrete structures due to the corrosion of steel reinforcement requires innovative solutions. In the 1970s and 1980s, it was a commonly accepted view that epoxy coatings would provide superior corrosion protection for reinforcing steel in concrete. For that reason, epoxy coated reinforcement was routinely used in North American bridge decks and substructures. In addition to epoxy coated reinforcement, cathodic protection systems have also been used to protect bridge substructures.
However, increasing concrete cover depth increases both dead load and construction costs and is generally unnecessary for structural reasons. Additionally, while epoxy coatings limit the exposure of the steel to chlorides, oxygen, and moisture and add minimally to bridge construction costs, breaks in the epoxy coating at cracked locations, in combination with high chloride concentrations, may result in corrosion of the steel reinforcement, which affects the overall performance of the bridge. Moreover, epoxy coatings in aging bridge decks may become brittle and eventually delaminate from the steel reinforcement.
CORROSION PRINCIPLES
The epoxy coating is intended to serve as a physical barrier to protect the reinforcing steel. Concrete normally provides a passive, electrically neutral film around the steel because of the high pH of the concrete. However, when chlorides in the presence of water and oxygen, penetrate the concrete, the pH is reduced and the passive film is destroyed. This allows the corrosion reaction to start at the steel surface. As diagrammed in Figure 1, the corrosion reaction consists of a simultaneous cathodic reaction and anodic reaction. The steel acts as an electrode that couples the two reactions.
Anodic Reaction
In the anodic reaction, iron atoms lose electrons (oxidation) because the passive film has been destroyed.
Fe › Fe2 + 2e-
The iron ions further react with the hydroxides to produce ferrous hydroxide.
Fe2 + 2OH- › Fe(OH)2
Ferrous hydroxide reacts in the presence of the diffused oxygen and this results in the production of rust, the final corrosion product at the anodic site.
4Fe(OH)2 + O2 › 2Fe2O3(rust) + 4H2O
Cathodic Reaction
Electrons flow through the steel where they react with water and oxygen to form hydroxide ions.
4e- + 2H2O + O2 › 4OH-
In the cathodic reaction, oxygen atoms are reduced and form hydroxide ions that further sustain the anodic reaction.
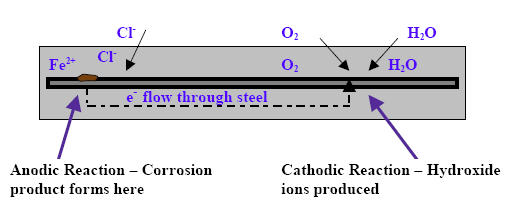
Figure 1: Corrosion reaction
The corrosion process is a natural electrochemical process where electron flow occurs in the steel and at the same time, hydroxide ions are conducted through an electrolyte, which in this case, is the pore water in the concrete. This completes an electric circuit. The completed circuit is known as a corrosion cell.
Two theories about the protection capability of epoxy coated reinforcement have been developed:
- Physical Barrier Theory – The epoxy coating acts as a barrier, preventing chloride ions and other aggressive matter from coming into contact with the steel surface.
- Electrochemical Barrier Theory – The epoxy coating acts as a high resistance coating, reducing corrosion by increasing electrical resistance between neighboring coated steel locations where the cathodic reactions can take place.
These two theories offer sound reasoning for the use of epoxy coated reinforcement as a protective measure for structures located in marine environments.
Fusion bonded epoxy coated reinforcement (FBECR) was developed in the late 1960 to early 70s in response to a severe rebar corrosion problems in bridge decks and is now a well established durability solution for protecting reinforced concrete structure in high chloride environments.
The early research evaluated different coating materials and concluded that fusion bonded epoxy powder coatings (FBECR) gave the best combination of properties for protecting rebar in chloride contaminated concrete. Subsequent laboratory and field studies have also shown that FBECR performs extremely well in comparison to unprotected steel reinforcement and galvanized.
FBECR is manufactured by applying specially formulated epoxy powder resins to blast cleaned and heated steel reinforcement. The heat in the reinforcement polymerizes the epoxy producing a robust coating that follows the reinforcement profile. Often FBECR is subsequently cut and bent to suit the requirement of the structural element. Non flexible FBE coatings are also used for improved bonding performance in which case the reinforcement is either pre-formed before coating or used in straight lengths. The method of application is summarized in Figure 2.

Figure 2: Method of manufacture of fusion bonded epoxy resin coated reinforcing bar
How FBECR protects
Corrosion of uncoated reinforcement is normally attributable to local loss of the of the passive surface film locally when chloride ions penetrate through the cover concrete, alternatively the local concrete alkalinity is reduced by carbonation of the concrete. The exposed steel then corrodes as an anode driven by the relatively large cathode of surrounding passive steel. Pitting results and is referred to as macrocell corrosion.
An epoxy coating on steel acts as a very effective barrier to aggressive agents, particularly chloride ions, which will not easily diffuse through a continuous epoxy coating. Hence the steel surface is protected.
Allowance must be made for some normal damage to the coating during assembly and concrete casting. The steel can corrode at any hole in the coating but the extent of corrosion is controlled by the surrounding intact epoxy layer which stifles the cathodic reactions and prevents macrocell corrosion. The steel surface at the hole has to sustain both the anode and the cathode and, because the cathodic reaction is much less efficient than the anodic reaction, the corrosion rate of the steel in the vicinity of the hole is 10 to 100 times less than it would be for uncoated reinforcement.
Performance of FBECR
There have been numerous research trials to evaluate the performance of FBECR. The conclusion is that it performs well and reduces the rate of deterioration of reinforced concrete containing high chloride levels. Furthermore, a comparison of the research work and the behavior of FBECR structures exposed to natural weathering shows that thousands of FBCER structures behave in accordance with the predictions of the laboratory based studies.
Some research projects have assessed the comparative performance of carbon steels, FBECR, galvanized rebar and stainless steel rebar. Invariable for chloride contaminated concrete, FBECR performs better than carbon steel and galvanized steel, but less well than austenitic stainless steel (Table 1).
Table 1: Comparison of cost and performance of different reinforcing bar materials in concrete
| Type of rebar | Relative cost | Corrosion protection method | Performance in carbonated concrete | Performance in concrete with high Cl | Other Factors |
| Carbon Steel | 1.0 | Cover concrete provides protection | Poor(Depends on concrete) | Poor(Depends on concrete) | Additional protection needed |
| Galvanized | 1.5 | Initially a barrier and subsequently sacrificial cathodic protection | Good | Poor | Reduced rebar ductility |
| FBECR | 2.0 | Barrier coating | Good | Good | Reduced bond to concrete |
| Austenitic Stainless Steel | 8.1 | Passive | Excellent | Excellent | Electrical Isolation form other rebar is required |
Access Precise Corrosion Properties Now!
Total Materia Horizon contains corrosion behaviour and property information for hundreds of thousands of materials, accross more than 2,000 media.

Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.