Production of Stainless Steel: Part Two
Abstract
Stainless steel production involves sophisticated melting and refining processes that can be classified into Duplex and Triplex routes. The Duplex process combines electric arc furnace melting with converter refining, while the Triplex process extends this by adding a VOD (Vacuum Oxygen Decarburization) plant for achieving ultra-low carbon, sulfur, and nitrogen contents. Currently, approximately 90% of global stainless steel production utilizes these advanced process routes. The AOD (Argon-Oxygen Decarburization) process has emerged as the dominant stainless steelmaking technology since the late 1960s, enabling precise control of carbon levels through mixed gas top-blowing techniques and argon dilution methods.
Understanding Stainless Steel Production Process Routes
The production of stainless steel involves multiple sophisticated process routes, each selected based on raw material availability, desired final product specifications, existing facility logistics, and capital economics. Modern stainless steelmaking processes are fundamentally categorized into two primary types: Duplex and Triplex refining systems.
The Duplex refining process represents a two-stage approach that combines electric arc furnace melting with subsequent converter refining. This established method provides excellent control over steel composition while maintaining operational efficiency. However, when applications demand extremely low levels of carbon, sulfur, or nitrogen in the final product, the Duplex process can be enhanced into a Triplex refining route through the installation of a VOD plant.
The Triplex process offers significant advantages over its Duplex counterpart, including increased productivity, higher daily heat capacity, improved scrap-to-liquid metal yield, enhanced metal quality, superior operational flexibility, and comparatively lower production costs. In the Triplex configuration, after initial melting in the primary unit, refining occurs in two separate vessels: the first vessel handles decarburization and major refining operations, while the second vessel manages final desulfurization and degassing processes.
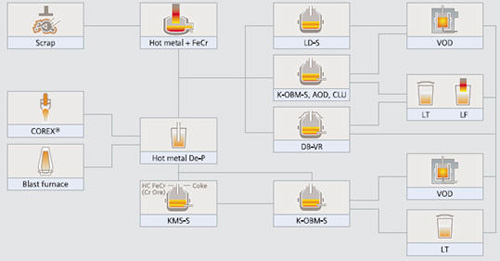
Figure 1: Standard process routes for stainless steels
Slag-Metal Reactions in Stainless Steel Refining
Understanding slag-metal reactions is crucial for optimizing stainless steel production efficiency. Research conducted by Pathy and Ward on CaO-MgO-SiO2-(Fe, Cr) oxide slags with lime/silica ratios between 1.2 and 2.0 revealed important solubility characteristics. The experimental solubility limit of Cr2O3 in slag varies significantly, ranging from 3% when in contact with low-chromium alloys (less than 2% Cr) to approximately 9% for alloys containing 14-20% chromium content.
These laboratory findings align with the established Cr2O3-CaO-SiO2 phase diagram determined by Glasser and Osborn. However, commercial slag operations demonstrate substantially higher chromium oxide solubility than laboratory results suggest. This discrepancy may result from several factors, including the solvent effect of alumina and iron oxide, the presence of suspended solid oxides, or the reality that slag and metal systems rarely achieve complete equilibrium in commercial operations.
Laboratory studies have established a linear relationship between the chromium ratio in slag versus metal and the FeO concentration in slag. The proportionality constant varies with temperature, ranging from 0.5 at 2950°F (1620°C) to 0.3 at 3070°F (1688°C). For commercial operations, the equation (Cr)SLAG / [Cr]METAL = 0.3 (%FeO) provides accurate representation of chromium partition ratios, enabling operators to determine chromium oxidation extent by measuring slag iron oxide concentration.
The AOD Process: Revolutionary Stainless Steel Production
The AOD (Argon-Oxygen Decarburization) process represents the most significant advancement in stainless steelmaking technology since its introduction in the late 1960s. This bottom-blown process has become the dominant method for producing high-quality stainless steel worldwide, utilizing mixed gas (O2 + inert gas) top-blowing lances to achieve exceptional decarburization rates even at extremely low steel bath carbon contents.
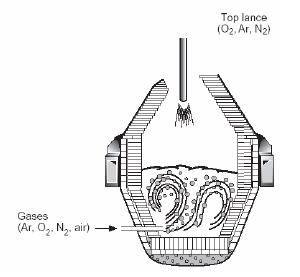
Figure 2: Schematic AOD Converter
The AOD converter receives molten steel and performs the critical task of refining crude steel with high carbon and sulfide content. The characteristically high carbon content undergoes oxidation and removal as carbon monoxide when oxygen flows through tuyeres positioned near the converter bottom. While chromium oxidation inevitably occurs during oxygen blowing decarburization, the resulting chromium oxide with its high melting point becomes enriched in the slag at steelmaking temperatures.
Depending on product specifications for carbon and sulfur content, operators reduce the top slag using ferrosilicon, aluminum, or a combination of both reducing agents. Ferrosilicon serves as the preferred choice when sulfur levels require only slight reduction or when sulfur content is already sufficiently low, primarily due to its cost-effectiveness compared to aluminum.
Advanced Decarburization Techniques in AOD Converters
The AOD converter achieves remarkably low carbon levels in stainless steel production, typically ranging from 0.01 to 0.04 percent. This exceptional capability stems from the innovative concept of introducing increasing amounts of argon into the oxygen blown through the steel bath during decarburization. The argon effectively dilutes the carbon monoxide formed during decarburization, thereby reducing the partial pressure of carbon monoxide (PCO).
Lower carbon monoxide partial pressure favors carbon monoxide formation from carbon and oxygen according to oxygen potential diagrams, enabling lower operating temperatures in AOD converters. For standard stainless steel containing 18% chromium and 0.03% carbon, normal atmospheric pressure (1 atm) requires temperatures of 1940°C to achieve such low carbon levels. However, when carbon monoxide partial pressure reduces to one-tenth of normal atmospheric pressure, the same low carbon content can be achieved at just 1600°C.
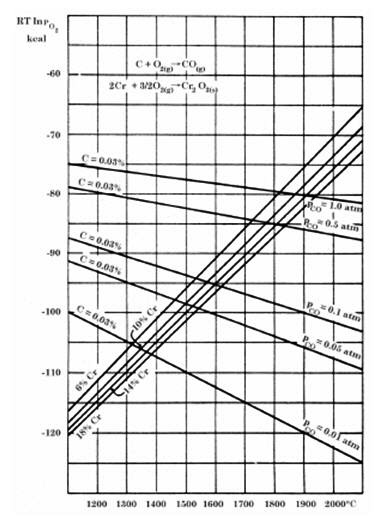
Figure 3: Oxygen potential diagrams
This temperature reduction provides substantial economic benefits, including shortened heating times, reduced heating costs, decreased refractory wear, and the potential use of less expensive refractories that don't require extreme temperature resistance.
Carbon Removal Efficiency and Process Optimization
Carbon Removal Efficiency (CRE) serves as a critical parameter for evaluating decarburization effectiveness, defined as the percentage of total inserted oxygen consumed in carbon oxidation: CRE = O2(carbon oxidation) / O2(total) x 100 (%).
The remaining oxygen oxidizes metal components, making high CRE maintenance crucial for process efficiency. When CRE decreases, operators must reduce the oxygen-to-argon ratio, requiring higher argon content in the blowing gas. This process occurs step-by-step with constantly increasing argon content.
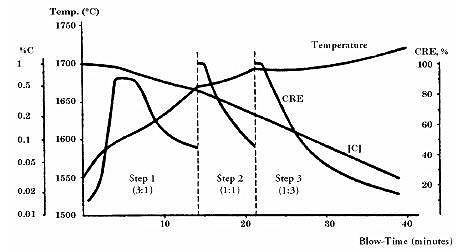
Figure 4: Chrome Reduction Efficiency CRE in AOD convertor
A typical decarburization procedure begins with CRE reaching 90%. As available carbon in the melt decreases, carbon removal efficiency declines. The second step, utilizing equal amounts of oxygen and argon, initiates when CRE approximates 45% and carbon level reaches 0.44%. During this phase, CRE rises to 100%, with all oxygen consumed in carbon monoxide formation. After several minutes, CRE decreases again alongside carbon levels, necessitating a third step with even higher argon content. The process continues until desired carbon levels are achieved, potentially requiring a fourth step using 100% pure argon.
Managing Chromium Oxidation in Stainless Steel Production
During decarburization procedures, elements with high oxygen affinity—including silicon, manganese, chromium, and nickel—undergo removal alongside carbon. Since stainless steel contains high chromium and nickel concentrations, these valuable elements, along with iron, inevitably experience some degree of oxidation during decarburization.
Major chromium losses to slag in oxide form are economically unacceptable. Through argon dilution, carbon undergoes preferential oxidation before chromium, following these reaction sequences:
[C] + [O] ↔ (CO) 4[Cr] + 3(O2)(G) ↔ 2Cr2O3(S) Cr2O3S + 3[C]Fe ↔ 2Cr + 3CO(G)
The overall combined reaction:
2[Cr] + 3CO(G) ↔ Cr2O3(S) + 3[C]
Research by Fulton and Ramachandran demonstrates that chromium oxidation depends significantly on blowing procedures. Argon protection against chromium oxidation diminishes when gas injection occurs only shallow into the melt from overhead lances, causing immediate chromium oxidation instead of carbon monoxide formation.
Conversely, when gas mixtures are injected through tuyeres positioned near the converter bottom, solid chromium oxide particles formed by instant oxidation become more effective. As Cr2O3 particles ascend to the surface, they perform actual decarburization by contacting carbon during their upward journey, releasing oxygen and forming carbon monoxide. Chromium oxide particles completely freed from oxygen molecules re-emerge as chromium in the melt, while particles not contacting carbon end up in slag.
Slag Chemistry and Final Process Considerations
In slag systems, chromium exists in both Cr2O3 and CrO forms. CrO formation is favored by increased temperature, decreased oxygen potential, and reduced basicity. To maintain chromium in its trivalent state as Cr2O3, operators must maintain high basicity levels.
The intensive argon purging of steel baths facilitates nitrogen and hydrogen removal, with both gases following argon to the surface, resulting in extremely low residual levels in the final steel product. This comprehensive gas removal capability represents one of the AOD process's most significant advantages in producing high-quality stainless steel grades.
Access Precise Properties of Stainless Steels Now!
Total Materia Horizon contains property information for 120,000+ stainless steels: composition, mechanical and physical properties, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.