Steel Deoxidation: Part Two
Abstract
This article examines the complex processes of steel deoxidation, focusing on the use of manganese, silicon, and aluminum as primary deoxidizing agents. The paper explores the paradoxical phenomenon of reoxidation that occurs when deoxidizer concentration exceeds critical values. Through detailed analysis of deoxidation equilibria and inclusion formation, the article presents the thermodynamic relationships governing these reactions at various temperatures. Key findings include the superior effectiveness of combined Si-Mn deoxidation compared to using manganese alone, and the significant impact of aluminum in forming specific oxide inclusions that affect steel quality.
Introduction to Steel Deoxidation
In all steelmaking processes, except for the acid silicon reducing process, the steel must be deoxidized after reaching the specified carbon content. This critical step makes dissolved oxygen inactive and prevents further oxidation of carbon.
There are primarily three elements used in steel deoxidation:
- Manganese and silicon (as low and high carbon ferro alloy or as silicomanganese alloy)
- Aluminum, approximately 98% purity
Fundamentals of Manganese Deoxidation
When steel is partially deoxidized with manganese, iron also participates in the reactions, forming liquid or solid Mn(Fe)O as the deoxidation product:
[Mn] + [O] → MnO
[Fe] + [O] → FeO
The state of equilibrium of steel with the deoxidation product Mn(Fe)O is illustrated in Figure 1 below.
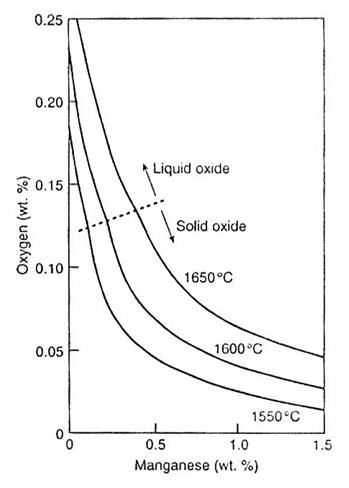
Figure 1: Manganese and oxygen contents of iron in equilibrium with FeO-MnO liquid or solid solution
Silicon-Manganese Combined Deoxidation
Deoxidation by silicon is significantly more complete than by manganese alone. Simultaneous deoxidation using both elements produces much lower residual oxygen in solution due to reduced silica activity. Depending on the concentration of Si and Mn added to steel in the tap ladle, the deoxidation product will be either molten manganese silicate (MnO·SiO₂) or solid silica (SiO₂).
[Si] + 2[O] → SiO₂ (1)
[Mn] + [O] → MnO (2)
One of the pioneering studies of slag-metal reaction equilibria was conducted by Korber and Oelsen, who measured the equilibrium distribution of manganese and silicon between liquid iron and MnO-FeO-SiO₂ slag saturated with silica. Their experimental results at 1600 ±10°C are shown in Figure 2.
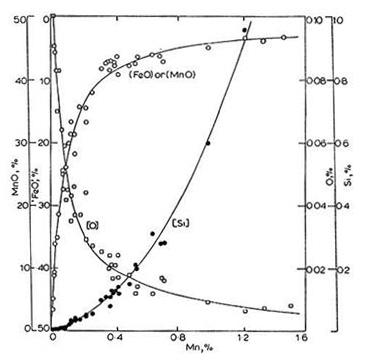
Figure 2: Concentration of Mn, Si and O in liquid iron equilibrated with SiO₂. Saturated manganese silicate melts at 1600 ±10°C
The equilibrium constant for Si deoxidation is expressed in equation (1). The following equilibrium relation is obtained for the Si-Mn deoxidation reaction:
KSi = aSiO₂ / [%Si] [%O]² (1a)
where the silica activity aSiO₂ is with respect to solid SiO₂ as the standard state.
The sum of the deoxidation reactions by silicon and manganese gives the following equilibrium relation:
[Si] + 2(MnO) = 2[Mn] + (SiO₂) (3)
KMnSi = {[%Mn]/aMnO}² a aSiO₂/[%Si] (3a)
log KMn-Si = 1510/T + 1.27 (3b)
where aSiO₂ and aMnO are relative to pure solid oxides. For high concentration of silicon (Si>0.4%), the activity coefficient fSi = 0.11 × [%Si].
The activities of MnO in manganese silicate melts have been measured by Rao and Gaskell. Their results substantially agree with earlier work by Abraham et al. The activity coefficients of the oxides (relative to solid oxides) are plotted in Figure 3.
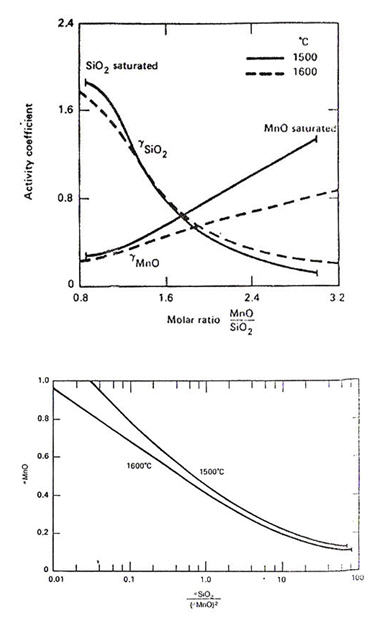
Figure 3: Activities in MnO-SiO₂ melts with respect to solid oxides, derived from the experimental data
For liquid steel containing Mn>0.4%, the deoxidation product is an MnO-rich silicate with FeO<8%. Therefore, the activity data in Figure 3 can be used together with equations (1) and (2) to compute the equilibrium state of the Si/Mn deoxidation as shown in Figure 4a. Whether the deoxidation product is solid silica or molten manganese silicate depends on temperature, Si and Mn contents, as illustrated in Figure 4b.
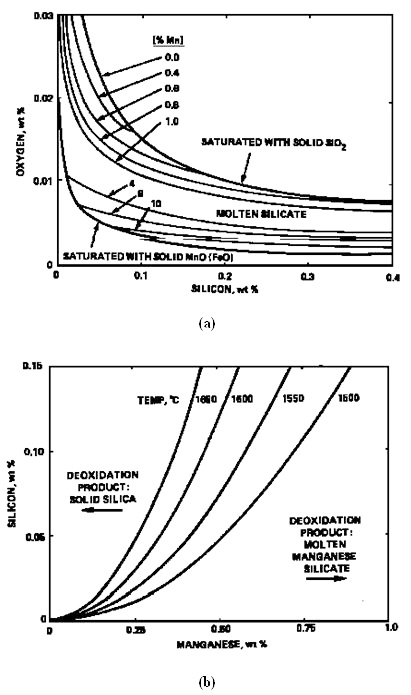
Figure 4a & 4b: Equilibrium relations for deoxidation of steel with silicon and manganese at 1600°C
Advanced Deoxidation with Silicon, Manganese, and Aluminum
Semi-killed steels with residual dissolved oxygen in the range of 40 to 23 ppm are produced by deoxidizing steel in the tap ladle with a small amount of aluminum together with silicomanganese or a combination of ferrosilicon and ferromanganese. In this case, the deoxidation product is molten manganese aluminosilicate with a composition similar to 3MnO Al₂O₃ 3SiO₂.
With small aluminum additions (approximately 35 kg for a 220 to 240 t heat) alongside Si/Mn, nearly all the aluminum is consumed in this combined deoxidation process. The residual dissolved aluminum in the steel will typically be less than 10 ppm.
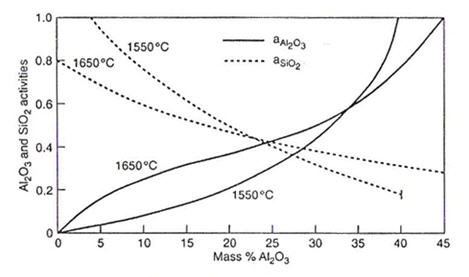
Figure 5: Al₂O₃ and SiO₂ activities in MnO-Al₂O₃-SiO₂ system for mass ratio of MnO/SiO₂=1
As shown in Figure 5, for the deoxidation product MnO-Al₂O₃-SiO₂ saturated with Al₂O₃, the silica activities are 0.27 at 1650°C, 0.17 at 1550°C, and decreasing to approximately 0.12 at 1500°C. Using these activity data and equation (1), the deoxidation equilibria are calculated for Al/Si/Mn and compared in Figure 6 with the residual oxygen [O] derived in ppm for the Si/Mn deoxidation at identical concentrations of Mn and Si.
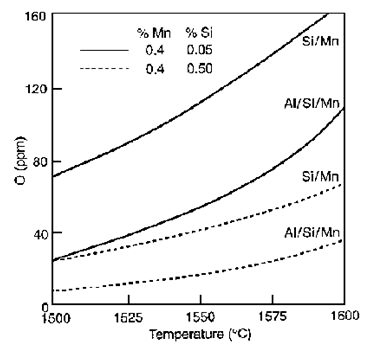
Figure 6: Deoxidation equilibria with Si/Mn compared with Al/Si/Mn for the deoxidation product saturated with Al₂O₃
Aluminum Deoxidation: Thermodynamics and Inclusions
Aluminum is an exceptionally effective deoxidizer used in most steelmaking operations. Aluminum deoxidation is typically carried out in the ladle; in special cases, aluminum additions are also made in the mold during ingot or continuous casting.
The equilibrium constants obtained from independent experimental studies agree within a factor of two. An average value for the equilibrium constant is given below:
Al₂O₃(s) = 2[Al] + 3[O] (4)
K = [%Al]² [ppm O × fO]³/ aAl₂O₃ (4a)
log K = -62,680/T + 31.85 (4b)
The alumina activity is with respect to pure solid Al₂O₃. The effect of aluminum on the activity coefficient of oxygen dissolved in liquid steel is given by log fO = -3.9 × [%Al]. At low aluminum concentrations, fAl = 1.0. Apparent equilibrium relations for the deoxidation products—pure Al₂O₃ and molten calcium aluminate with %CaO/Al₂O₃ = 1:1—are shown in Figure 7.
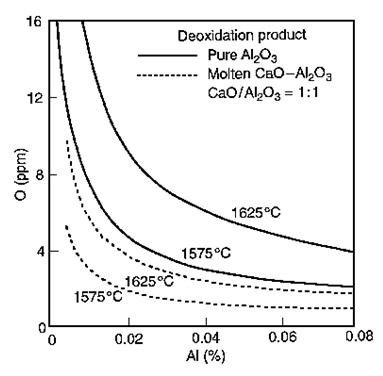
Figure 7: Deoxidation with aluminum in equilibrium with Al₂O₃ or molten calcium aluminate with CaO/Al₂O₃ = 1:1
When Al-killed steel is treated with Ca-Si, alumina inclusions convert to molten calcium aluminate. For the ratio %CaO/Al₂O₃ = 1:1, the activity of Al₂O₃ is 0.064 with respect to pure Al₂O₃ at temperatures ranging from 1500°C to 1700°C.
Read more
Access Precise Properties of Structural Steels Now!
Total Materia Horizon contains property information for 150,000+ structural steels: composition, mechanical and physical properties, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.