Multi Phase Twinning-Induced Plasticity (TWIP) Steel
Abstract
The iron-manganese TWIP steels, which contain 17-20% of manganese, derive their exceptional properties from a specific strengthening mechanism: twinning. The steels are fully austenitic and nonmagnetic, with no phase transformation. The formation of mechanical twins during deformation generates high strain hardening, preventing necking and thus maintaining a very high strain capacity.
The iron-manganese TWIP steels, which contain 17-20% of manganese, derive their exceptional properties from a specific strengthening mechanism: twinning. The steels are fully austenitic and nonmagnetic, with no phase transformation. The formation of mechanical twins during deformation generates high strain hardening, preventing necking and thus maintaining a very high strain capacity.
It is well known that the properties of different steels are determined by their crystal lattice structures, that is the spatial arrangement of their atoms. Adding alloying elements makes certain crystal structures more likely to form which allows the properties of the steel to be fine-tuned. It is concluded from thermodynamic calculations that a combination of manganese, silicon and aluminum would probably be suitable for the development of the new lightweight construction steel. These elements are lighter than iron and they force the crystal lattice into certain structures: iron can switch between different crystal lattices, or iron atoms can switch their positions and form different arrangements in the crystal lattices.
There is, for example, an f.c.c.: face-centered cubic arrangement, known as "austenite". In this case, the iron atoms sit on the corners of the crystal lattice cube with an atom in the center of each face of the cube. Then there is the b.c.c.: body-centered cubic layout. Again, the iron atoms are arranged on the corners, but with another one in the cube's center. There is also a type in which the iron atoms are distributed in a hexagonal arrangement. The body-centered cubic and the hexagonal forms are both traditionally referred to as martensite. The crystal lattice changes, and with it, the character of the steel, depending on the alloy element content (the alien atoms in the crystal lattice).
Conventional high strength steels were manufactured by adding the alloying elements such as Nb, Ti, V, and/or P in low carbon or IF (interstitial free) steels. These steels can be manufactured under the relatively simple processing conditions and have widely been applied for weight reduction.
However, as the demands for weight reduction are further increased, new families of high strength steel have been developed. These new steels grades include DP (dual phase), TRIP (TRansformation Induced Plasticity), FB (ferrite-bainite), CP (complex phase) and TWIP (TWin Induced Plasticity) steels.
The critical part of the steel manufacturing steels is to control the processing parameters so that the microstructure and, hence, the strength-elongation balance could be optimized. Various high added value products are developed to satisfy increasing customer demands, as shown in Figure 1.
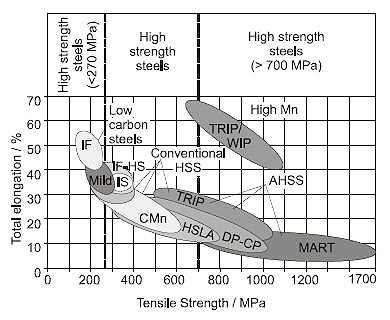
Figure 1: Ductility-strength relationship of mild and high strength steels.
Recently, new group of austenitic steels with 15-25 percent of manganese contents and 3 percent of aluminum and silicon has been developed for automotive use. This group is divided into transformation induced plasticity steels (HMS-TRIP) and twinning induced plasticity steels (HMS-TWIP) due to the characteristic phenomena occurring during plastic deformation inside the grains.
At 700 MPa, the TRIP steels are also exceptionally strong. However, their ductility is moderate, at approximately 35 percent. This characteristic – ductile yet strong – is the result of changes in the crystal lattice. When forces act on the steel, it changes from the face-centered cubic form – austenite – to the body-centered cubic form – martensite. It is the collective shear of the crystal lattice planes (the transformation) that makes traditional TRIP steel ductile.
However, with conventional TRIP steel, a certain amount of the austenite portion is transformed to martensite – a rigid crystal structure that allows hardly any stretching. In crash tests, this steel offers only about 5 percent additional ductility.
With the increased share of manganese, silicon and aluminum atoms in the iron crystal, the TRIP effect is twice as profound, thus providing double additional ductility. The reason for twinning is that the alloy elements make two martensitic transformations possible – first a change from austenite to hexagonal martensite, and then from the hexagonal structure to the body-centered cubic martensite.
The twinning causes a high value of the instantaneous hardening rate (n value) as the microstructure becomes finer and finer. The resultant twin boundaries act like grain boundaries and strengthen the steel. TWIP steels combine extremely high strength with extremely high formability. The n value increases to a value of 0.4 at an approximate engineering strain of 30% and then remains constant until a total elongation around 50%. At the same time, it hardens without breaking and it resists tensile pressures up to 1100 MPa and it could be stretched to approximately 90 percent of its length without breaking (Figure 2).
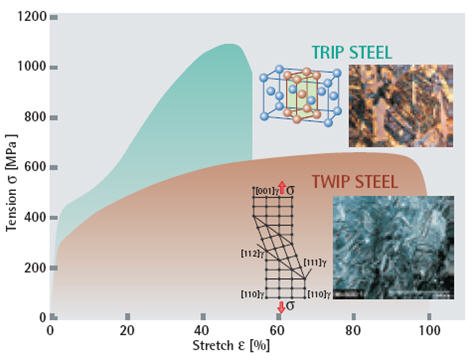
Figure 2: The stress-strain diagram clearly shows the differing characters of TRIP and TWIP steel. TRIP steel can resist high stresses without deforming. TWIP steel deforms with low stresses, but does not break until strain reaches around 90 percent.
It is means in practice that when forces act on the steel, as in the deep draw process, some of the austenite first transforms to the first martensite stage, the hexagonal crystal form. When the steel is put under increasing stress, the hexagonal lattice switches to the final, body-centered cubic form, similar to conventional TRIP steel. This means that the steel retains a good part of its ductility even after deep draw processing.
Also, the TRIP steel is particularly useful for side impact protection. The material deforms and absorbs the energy of the impact. It also becomes very strong as it hardens, which prevents the side sections from collapsing too much and protects vehicle occupants from injury.
However, the double TRIP effect does not explain why an alloy with 15-25 manganese content is particularly ductile. This is caused by small faults in the crystal structure called "stacking faults". Stacking faults can be visualized as a shift in the grid of atomic planes neatly arranged side by side and one on top of the other. If an extra stack of two atomic planes is introduced into the lattice from above, the regular stacking sequences are disturbed and therefore form a stacking fault. This folding mechanism takes place on a mirror plane, creating regularly mirrored sections of crystal. Experts refer to this as twinning, which is what manifests itself externally as extreme ductility.
Typical mechanical property ranges of these different steels are indicated in Figure 3. It is obvious that High Manganese Steels show extraordinary strength-ductility relationships with a resist tensile stress up to 1100 MPa. Conventional high-strength bodywork steels rupture at around 700 MPa or even less.
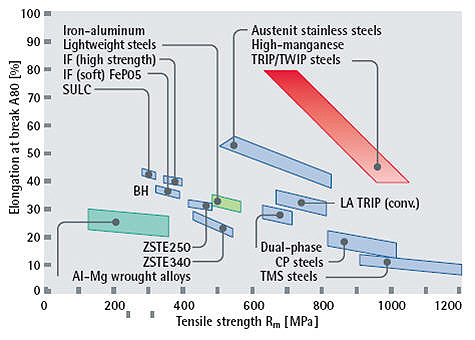
Figure 3: The diagram shows the very high stresses that TRIP/TWIP steels (red) can resist, compared to conventional deep drawing steels (blue).
High manganese steels composed of single austenite phase or multi phase with high fraction of austenite phase can be alloyed with a large amount of alloying elements. Effect of alloying element on properties of high manganese steels is shown in Table 1.
| Element | γ-stabiliser | Solid solution strengthening austenite | ε-matrensite refinement | Hot ductility |
| C | + | + | ||
| Mn | + | |||
| Si | + | + | ||
| B | + | |||
| Ti | + | |||
| N | + | + |
As discussed above, carbon improves the stability of austenite and strengthens the steels. It inhibits the formation of ε-martensite by increasing the stacking fault energy. Manganese stabilizes austenite. However if its content is less than 15%, α'-martensite is formed, which aggravates the formability.
The γ => ε transformation temperatures decrease with increasing Mn content. Silicon improves strength by solid solution strengthening. Silicon addition is effective for refining ε martensite plates and increasing fracture strength, although it does not improve ductility. The high aluminum content in high manganese steels increases the stacking fault energy of austenite. The formation of ε-martensite is suppressed by aluminum addition. An aluminum addition is also very effective for improving of low temperature toughness. Aluminum can segregate on the grain boundaries during solidification, and produce a low melting point intermetalic compound such as Fe2Al5 having a melting point about 1170°C on the grain boundaries, which cause a weakness in the casting structure.
Adding small amounts of boron, titanium and zirconium into the high manganese steels HMS alloyed with aluminum can improve the hot ductility of the steels. Nitrogen is an effective strengthening element in austenite e.g. adding nitrogen to the Fe16.5Mn alloy decrease the martensite start temperature and also reduces the volume fraction of ε-martensite.
TWIP steels have very good mechanical advantages for the improvement of the automotive design, a very good crash resistance and they also reduce the vehicle weight. This new class of steels is a good example of the development of new materials for the benefit of the human being.
Find Instantly Precise Material Properties!
Total Materia Horizon contains mechanical and physical properties for hundreds of thousands of materials, for different temperatures, conditions and heat treatments, and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.