Effects on the martensite, pearlite and bainite formation
Abstract
All alloying elements with the possible exception of Co, lower temperature of the start of the martensite formation, as well as the finish of the martensite formation, i.e. at 100% martensite.
All alloying elements except Co delay the formation of ferrite and cementite. It is very difficult to formulate any general rules regarding the influence exerted by the various alloying elements. However, it has definitely been found that some elements affect the bainite transformation more than the pearlite transformation, while other elements act in the opposite manner.
Effect on the temperature of martensite formation
All alloying elements with the possible exception of Co, lower Ms the temperature of the start of the martensite formation, as well as Mf, the finish of the martensite formation, i.e. at 100% martensite. For the majority of steels containing more than 0,50% C, Mf lies below room temperature.
This implies that after hardening these steels practically always contain some residual austenite. Ms may be calculated from the equation given below, by inserting the percentage concentration of each alloying element in the appropriate term. The equation is valid only if all the alloying elements are completely dissolved in the austenite.
Ms = 561 - 474C - 33Mn - 17Ni - 17Cr - 21Mo
For high-alloy and medium-alloy steels Stuhlmann has suggested the following equation:
Ms(°C) = 550 - 350C - 40Mn - 20Cr - 10Mo - 17Ni - 8W - 35V - 10Cu + 15Co + 30Al
It can be noted that carbon has the strongest influence on the Ms temperature. Figures 1 and 2 show diagrams with an example of experimental results of the effect of Mn and Ni on the Ms temperature of various types of steel.
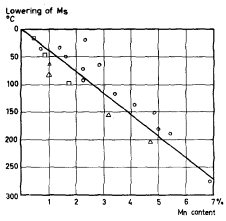
| Figure 1. | Effect of Mn on the Ms - temperature (after Russel and McGuire, Payson and Savage, Zyuzin, Grange and Stewart) |
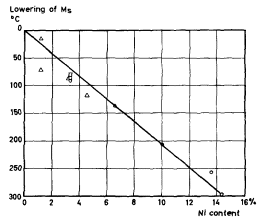
| Figure 2. | Effect of Ni on the Ms - temperature (after Russel and McGuire, Payson and Savage, Zyuzin, Grange and Stewart) |
Effect on the formation of pearlite and bainite during the isothermal transformation
All alloying elements except Co delay the formation of ferrite and cementite. It is very difficult to formulate any general rules regarding the influence exerted by the various alloying elements. However, it has definitely been found that some elements affect the bainite transformation more than the pearlite transformation, while other elements act in the opposite manner.
Certain elements will, paradoxically, accelerate the transformations if their concentration increases beyond a certain limiting value, this limit been affected by other alloying elements present. For case-hardening and tool steels the time taken to initiate the pearlite-bainite transformation is reduced as the carbon content exceeds about 1%. For tool steels and constructional steels Si-concentrations of 1,5% and above have been found to promote pearlite formation.
As a general principle it may be stated that by increasing the concentration of one alloying element by some few percent and the basic carbon content being kept about 0,50%, only a relatively small retardation of the transformation rates is noticed. For plain carbon steels a successive increase in C from 0,30% to 1% produces but a negligible effect. It is only in conjunction with several alloying elements that a more noticeable effect is produced.
The diagram in Figure 3, applicable to steel W 1 (l% C) will serve as a basis for this discussion. The shortest transformation time for this steel is less than 1/8th second. Note that the time scale is logarithmic; hence there is no zero time. As has been mentioned previously, both pearlite and bainite form simultaneously in this steel at about 550°C. Since the curves overlap it is customary to draw only one curve. With increasing contents of certain alloying elements, however, the noses of the pearlite and bainite curves will separate.
The structures shown in Figure 3 are obtained by austenitizing samples of steel W 1 at 780°C for 10 min and quenching in a salt bath at various temperatures. After holding them for predetermined times at various temperatures they are finally quenched in water. Before the salt-bath quenching the steel contains undissolved carbides but in view of the composition of the austenite the steel may be regarded as an eutectoid one. The diagram should be studied with the aid of the explanatory text below.
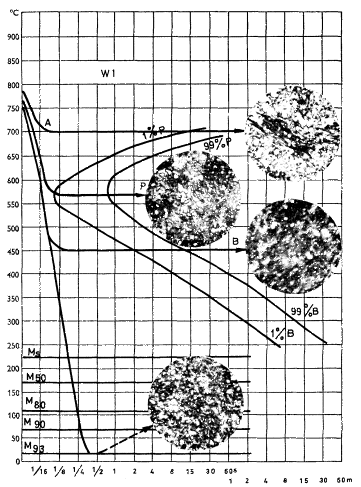
| Figure 3. | TTT diagram for isothermal transformation of steel W 1 (1% C) A = austenite, B = bainite, Ms = start of martensite transformation, M50 = 50% M, P = pearlite |
- Quenching in a liquid bath at 700°C; holding time 4 min. During this interval the C has separated out, partly as pearlite lamellae and partly as spheroidized cementite. Hardness 225 HV.
- Quenching to 575°C, holding time 4 s. A very fine, closely spaced pearlite as well as some bainite has formed. Note that the amount of spheroidized cementite is much less than in the preceding case. Hardness 380 HV.
- Quenching to 450°C, holding time 60 s. The structure consists mainly of bainite. Hardness 410 HV.
- Quenching to 20°C (room temperature). The matrix consists of, roughly, 93% martensite and 7% retained austenite. There is some 5% cementite as well which has not been included in the matrix figure. Hardness 850 HV.
Find Instantly Thousands of Metallography Diagrams!
Total Materia Horizon contains a unique collection of metallography images across a large range of metallic alloys, countries, standards and heat treatments.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.