Low Phosphorus Partition Ratio Models
Abstract
Phosphorus is a key impurity which affects predominantly the ductility of the finished product and so it is essential that methods are employed to keep the levels of phosphorus in a controlled and desirable range.
General control of phosphorus content is carried out by a plant specific dephosphorization process which is a model that is honed by continuous monitoring and adjustment.
A real steelmaking process involves many complex physical and chemical coupled phenomena such as oxidation, decarburization, dephosphorization, and slag formation.
Phosphorus is an element of representative impurities that should be kept as low as possible in conventional steel grades. Low phosphorus content steels are essential for steel applications where high ductility is required, such as thin sheets, deep drawn structures, pipelines, and automobile exteriors. Phosphorus’ ability to strengthen and embrittle ferrite imposes restrictions on the maximum phosphorus content for the aforementioned applications. The main source of phosphorus in steel is from the raw materials; thus, raw material selection is a way to maintain the phosphorus content within the desirable range.
In (BOF) oxygen steelmaking it is difficult to predict the distribution of phosphorus between slag and metal by direct application of models (listed in Table 1), even though a pseudo-steady state is usually attained between slag and metal towards the end of the blow. This is because under practical (shop-floor) conditions, besides the slag composition and temperature, the phosphorus distribution is found to depend also upon other parameters, such as slag mass, turndown carbon, initial phosphorus content of metal, intensity of bottom stirring, lance height, oxygen flow rate, addition scheme and timing of addition of fluxes and iron ore.
Therefore, each plant usually develops its own blowing practice and, on the basis of analysis of plant data, adapts a suitable model for control and prediction of phosphorus at tap. The selection of a control model is not a simple task. It is to be noted that the models listed in Table I do not explicitly take into account the effect of precipitation and/or dissolution of C2S on phosphorus distribution.

Table 1: Phosphorus Distribution log Lp Equations
The efficiency of dephosphorization process may be given by the distribution coefficient for phosphorus between slag and the metal phase LP = (%P2O5)/[%P], and dephosphorization degree, which is equal to the ratio of the phosphorus concentration removed from the metal to the initial phosphorus concentration ηP = [%∆P]/[%Pini].
On the other hand, it is well known that the phosphorous distribution between slag and metal (LP) is generally lower (10 to 50) for EAF steelmaking than for oxygen steelmaking (50 to 200).
The phosphorus [P] removal in BOS steelmaking is generally described by the ionic reaction shown in equation (1). From (1) it can be seen that a high [P] activity, high oxygen potential [O] and high basicity O2- all promote phosphorus removal. Also, [P] removal may also be represented by the simple molecular reaction, given in equation (2), allowing a Gibbs free energy to be calculated using equation (3).
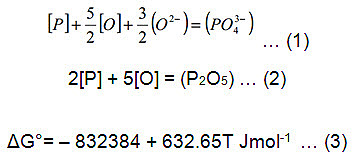
The equilibrium constant of this reaction is given by equation (4)
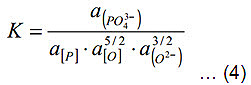
It is generally agreed that LP increases with slag basicity and oxygen activity and decreases with increasing temperature.
Find Instantly Thousands of Heat Treatment Diagrams!
Total Materia Horizon contains heat treatment details for hundreds of thousands of materials, hardenability diagrams, hardness tempering, TTT and CCT diagrams, and much more.

Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.