Clean Steel: Part Two
Abstract
Non-metallic inclusions are undesirable components present in all steels that significantly impact steel properties and quality. Controlling these inclusions is fundamental to the concept of "clean steel" production and plays a crucial role in maintaining steel's competitive advantage over newer materials. This article examines the classification, formation mechanisms, and effects of non-metallic inclusions on steel properties. The primary types include oxides and sulfides, which generally have negative effects on mechanical properties by initiating fractures. However, some inclusions can benefit steel properties by nucleating acicular ferrite during phase transformation. Understanding inclusion behavior during plastic deformation is essential for optimizing final product properties through effective inclusion control strategies.
Introduction to Non-Metallic Inclusions in Clean Steel Production
Non-metallic inclusions represent one of the most significant challenges in modern steel manufacturing. These undesirable components, present in all steels to varying degrees, substantially influence the final properties of steel products. The concept of "clean steel" is intrinsically linked to the effective control and management of these inclusions, making their understanding crucial for metallurgists and steel producers worldwide.
The improvement of steel properties through systematic control of non-metallic inclusions has become increasingly important as the steel industry faces competition from newer materials. Contemporary metallurgical practice aims to eliminate harmful inclusions while controlling the nature and distribution of remaining inclusions to optimize the properties of the final steel product.
Understanding Steel Cleanliness and Inclusion Distribution
The assessment of steel cleanliness presents unique challenges in metallurgical evaluation. All steels contain non-metallic inclusions to varying extents, and the concept of steel cleanliness remains relative. Even steel containing as little as 1 ppm each of oxygen and sulfur will still contain between 10⁹ to 10¹² non-metallic inclusions per ton. From this perspective, all steels can be considered "dirty" to some degree.
The type and morphology of non-metallic inclusions depend on several critical factors including steel grade, melting process, secondary metallurgy treatments, and casting methods. This variability makes the determination of steel purity particularly significant in quality control processes.
Effects of Non-Metallic Inclusions on Steel Properties
Non-metallic inclusions in steel typically contribute negatively to mechanical properties, primarily because they can initiate both ductile and brittle fracture mechanisms. These inclusions serve as stress concentration points and crack initiation sites, leading to various material defects including brittleness and diverse crack formations.
However, the relationship between inclusions and steel properties is not entirely negative. Certain inclusions can provide beneficial effects by nucleating acicular ferrite during the austenite-to-ferrite phase transformation, particularly in low-carbon steels. This dual nature of inclusion effects highlights the importance of controlled inclusion management rather than complete elimination.
Comprehensive Classification of Non-Metallic Inclusions
Chemical Classification Systems
Non-metallic inclusions are systematically classified based on their chemical composition, stability characteristics, and origin. The primary chemical categories include:
- Oxide Inclusions: These represent the most common type of inclusions, ranging from simple oxides such as FeO, MnO, Cr₂O₃, TiO₂, SiO₂, and Al₂O₃ to complex compound oxides including FeO·Fe₂O₃, FeO·Al₂O₃, MgO·Al₂O₃, and FeO·Cr₂O₃.
- Sulfide Inclusions: This category encompasses simple sulfides like FeS, MnS, CaS, MgS, and Al₂S₃, as well as compound sulfides such as FeS·FeO and MnS·MnO.
- Nitride Inclusions: Typically found in alloyed steels containing strong nitride-forming elements, these include simple nitrides like TiN, AlN, ZrN, and CeN, along with complex nitrides such as Nb(C,N) and V(C,N).
- Phosphide Inclusions: Less common inclusions including Fe₃P and Fe₂P.
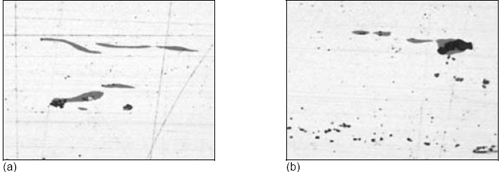
Figure 1: Typical appearance of sulfides (light gray) and oxides (dark gray) in steel microstructure
Mineralogical Classification of Oxide Inclusions
Oxide inclusions can be further categorized based on their mineralogical characteristics:
- Free Oxides: Including simple compounds such as FeO, MnO, Cr₂O₃, SiO₂ (quartz), and Al₂O₃ (corundum).
- Spinel Compounds: Complex oxides formed by bi- and tri-valent elements, including ferrites, chromites, and aluminates.
- Silicate Inclusions: Present as either glass-like formations of pure SiO₂ or SiO₂ combined with iron, manganese, chromium, aluminum, and tungsten oxides, as well as crystalline silicate structures.
Sulfide Inclusion Morphology and Formation Mechanisms
Sulfide inclusions in steel require particular attention due to their significant impact on the steelmaking process and final product properties. Their behavior during steel working processes, especially their morphological characteristics, substantially affects steel properties.
Research analysis of steel ingots containing 0.01-0.15% sulfur has identified three distinct morphological types of MnS inclusions:
- Type I MnS: Characterized by globular morphology with a wide size distribution, often forming duplex structures with oxide inclusions.
- Type II MnS: Features dendritic structure and is commonly referred to as grain-boundary sulfide due to its distribution as chain-like formations or thin precipitates along primary ingot grain boundaries.
- Type III MnS: Exhibits angular morphology and consistently forms as single-phase inclusions.
The formation of these sulfide types occurs during secondary metallurgy processes or solidification. Modern steelmaking technology has dramatically reduced sulfur concentrations in steel, while continuous casting technology with higher cooling rates has largely replaced traditional ingot casting methods.
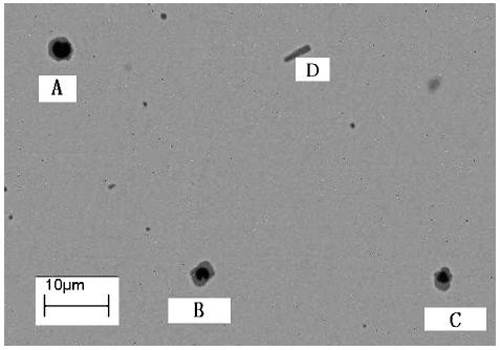
Figure 2: Typical morphology of MnS in conventional continuous casting steel, showing both globular duplex oxide-sulfide particles (A, B, and C) and Widmanstätten plate-like MnS (particle D)
Advanced Modeling of Inclusion Behavior During Steel Processing
Understanding the behavior of non-metallic inclusions and their surrounding matrix during plastic working processes is crucial for optimizing steel properties. Advanced computational modeling using programs such as ABACUS has enabled detailed analysis of slag inclusion behavior during hot rolling and hot forging of hardenable steels.
These modeling studies reveal critical insights that are difficult or impossible to obtain through conventional experimental methods. The computational analysis demonstrates how inclusions interact with the surrounding matrix material under various processing conditions.
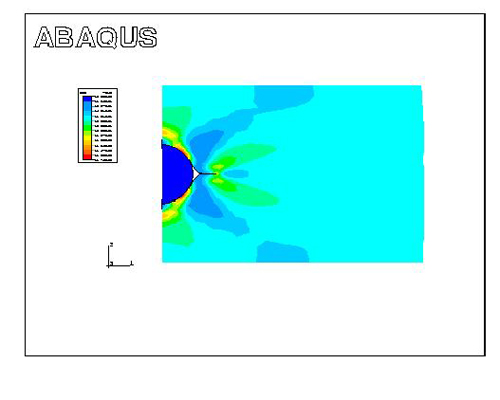
Figure 3: Effective strain contour during plastic deformation, showing three regions of strain concentration and the formation of a trihedral void near round inclusions
The modeling results indicate that differences in mechanical properties between the matrix and inclusions are the primary factors in void formation. Weak bonding at the matrix-inclusion interface facilitates void opening, creating potential failure initiation sites.
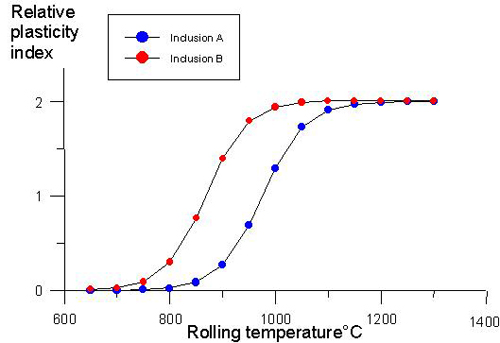
Figure 4: Effects of rolling temperature on the relative plasticity index during hot rolling operations
The relationship between rolling temperature and inclusion plasticity shows a clear transition region where the relative plasticity index changes rapidly. This finding aligns with existing experimental observations and provides valuable guidance for optimizing hot rolling parameters.
Conclusion and Future Directions in Clean Steel Technology
The control of non-metallic inclusions remains fundamental to achieving clean steel production and maintaining steel's competitive position in modern materials applications. Effective inclusion management requires comprehensive understanding of inclusion formation, classification, and behavior during processing.
Future developments in clean steel technology will likely focus on advanced inclusion engineering, where beneficial inclusions are deliberately controlled while harmful inclusions are minimized. This approach, combined with improved computational modeling capabilities, promises to deliver enhanced steel properties for increasingly demanding applications.
The metallurgist's ultimate goal remains the elimination of undesirable inclusions while controlling the nature and distribution of remaining inclusions to optimize final product properties. This balanced approach to inclusion management represents the future of clean steel production technology.
Read more
Find Instantly Precise Compositions of Materials!
Total Materia Horizon contains chemical compositions of hundreds of thousands materials and substances, as well as their mechanical and physical properties and much more.

Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.