Boron in Steel: Part Two
Abstract
Boron serves as a critical alloying element in steel, primarily recognized for its exceptional hardenability enhancement properties. This article explores how minute additions of boron (typically 3-15 ppm) can significantly improve steel hardness, providing effects comparable to larger quantities of more expensive alloying elements like nickel, chromium, or molybdenum. The paper examines the mechanisms behind boron's hardenability enhancement, its interaction with other elements, optimal processing conditions, and applications across various industries. Particular attention is given to boron's role in structural and automotive steels, where it enables the production of high-strength components while offering cost advantages over traditional alloy steels. The limitations of boron usage and considerations for proper heat treatment are also discussed.
The Fundamental Role of Boron in Steel Metallurgy
Boron is useful as an alloying element in many materials, but in this paper it will be illustrated as an alloying element in steel because of its remarkable effect on hardenability enhancement. Boron is added to unalloyed and low alloyed steels to enhance hardness levels through improved hardenability. When added to high-speed-cut steels, for example those containing 18% tungsten, 4% chromium, and 1% vanadium, boron enhances cutting performance while reducing forging qualities.
Addition of boron in quantities up to 0.01% to austenitic steels also improves their high-temperature strength. Boron steels are widely used as high-quality, heat-treatable constructional steels, steels for carburization, and cold forming steels such as those used for screws. The addition of 5 to 50 ppm boron to ferritic steels containing 14 to 18% chromium may improve the surface quality of stainless strips by avoiding defects such as scale, ribbing, roping, and ridging, which otherwise frequently occur in strip production.
Mechanism and Optimization of Boron's Hardenability Effect
The basic effect of boron in steel is the enhancement of hardenability, which is evident even at very small concentrations of around 0.0010% (10 ppm). Boron is added to unalloyed and low alloyed steels to enhance hardness levels through improved hardenability. Even in small quantities up to 100 ppm, boron provides the same hardenability enhancement as other more expensive elements that must be added in much larger quantities. For example, the addition of just 30 ppm of boron in SAE steels can replace approximately 1% nickel, 0.5% carbon, 0.2% manganese, 0.12% vanadium, 0.3% molybdenum, or 0.4% chromium.
The mechanism decisive for hardenability enhancement by boron is a delay in transformation to bainite, ferrite, and pearlite structures, which are softer than martensite. Unless prevented by boron, these softer structures would form during cooling from austenitization temperature after annealing or hot working. It is generally accepted that hardenability peaks when boron content is between 3 and 15 ppm. Excessive amounts of boron (>30 ppm) can cause segregation at austenite grain boundaries, which not only lowers hardenability but may also decrease toughness, cause embrittlement, and produce hot shortness. Boron's effect on hardenability also inversely correlates with the carbon content in the steel.
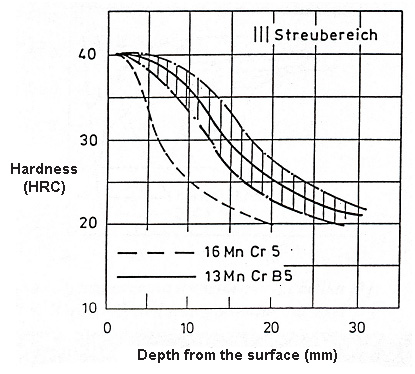
Figure 1: Hardenability curves of boron low alloyed steels (13MnCrB5) compared to steel without boron (16MnCr5)
Critical Processing Considerations for Boron-Enhanced Steels
Boron must be in its atomic state to improve hardenability, which requires careful attention during steel production to ensure effectiveness. Boron may become ineffective if its state is changed by incorrect heat treatment. For example, high austenitizing temperatures must be avoided, as well as temperature ranges where certain boron precipitates form.
Hardenability is highly dependent on the behavior of oxygen, carbon, and nitrogen present in steel. Boron reacts with oxygen to form boron oxide (B₂O₃), with carbon to form iron boroncementite (Fe₃(CB)) and iron boroncarbide (Fe₂₃(CB)₆), and with nitrogen to form boron nitride (BN). Loss of boron through oxidation is prevented by making boron additions to silicon-aluminum killed steels and by using good ladle and mold practices. Strong nitride formers such as titanium, aluminum, and zirconium protect boron from reacting with nitrogen. For instance, if nitrogen is fixed using titanium, satisfactory hardenability is obtained in temperatures up to 1830°F (1000°C), provided the steel contains about 5-20 ppm of boron.
The hardenability of boron steel is also closely related to austenitizing conditions and generally decreases when heated above 1830°F (1000°C). Additionally, boron steel must be tempered at lower temperatures than other alloy element steels of comparable hardenability to maintain its beneficial properties.
Commercial Applications and Industry-Specific Uses of Boron Steels
Boron steels are used for a variety of applications, both as wear-resistant materials and high-strength structural components. Examples include punching tools, spades, knives, saw blades, and safety beams in vehicles. Carbon-manganese-boron steels are generally specified as replacements for more expensive alloy steels while maintaining equivalent hardenability. Applications for these steels include earth scraper segments, track links, rollers, drive sprockets, axle components, and crankshafts.
Boron alloy steels are specified when the base composition meets mechanical property requirements (such as toughness and wear resistance), but hardenability is insufficient for the intended section size. Rather than using a more highly alloyed and therefore more expensive steel, users may simply specify the corresponding boron grade to ensure suitable hardenability.
An expanding area of boron usage is in high strength low alloy (HSLA) and other structural steels. These may be supplied as hot rolled or as quenched and tempered (the latter being more common for boron grades). Boron ensures adequate hardenability in heavier plate sections. In the automotive industry, boron steel has gained significant popularity, especially in European vehicles. The boron steel used in Volvo cars, for example, has an extremely high yield point of about 1,350-1,400 MPa (196,000-203,000 psi)—approximately four times stronger than average high-strength steel. This makes it ideal for safety-critical components such as bumper reinforcements, guard beams, B-pillar reinforcements, and body panels.
Boron is also used in non-heat treated steels and specialized applications like nuclear industry stainless steels, where its high neutron absorption capability is valuable. When properly tempered after oil or water quenching, boron steels possess hardness equivalent to much higher carbon steels and more expensive low alloy steels, while offering improved cold formability, better weldability, and good case hardening response.
Specialized Applications and Future Trends in Boron Steel Technology
Boron has found unique applications beyond traditional structural uses. In the nuclear industry, boron's high neutron absorption capability makes it valuable in certain types of stainless steel. While levels of up to 4% boron have been used, the reduced hot ductility and weldability at such concentrations means that boron contents of 0.5 to 1.0% are more common for neutron absorption applications. Even at these lower concentrations, the ferroboron must be of the highest purity to maintain desired properties.
Medium carbon steels with deliberate boron additions (such as Boron 922 with 0.25-0.30% carbon and Boron 921 with 0.38-0.42% carbon) show improved hardness during heat treatment. These steels, when toughened by tempering following oil or water quenching, possess hardness equivalent to much higher carbon steels and more expensive low alloy steels. The advantages of these boron steels include improved cold formability, lower delivered hardness giving improved blanking tool life, improved weldability due to low carbon equivalents, lower tempering temperatures providing energy savings, and good case hardening response. Common applications include toecaps and chains.
In automotive applications, boron steel continues to gain traction, particularly in safety-critical components. The exceptional strength of automotive boron steel presents unique challenges for repair and replacement, as the process that creates its strength reduces some workability properties, such as the ability to straighten it. As manufacturing techniques evolve and the demand for lightweight, high-strength materials increases, boron steel is likely to find even more applications across various industries where the balance of strength, formability, and cost efficiency is crucial.
Read more
Find Instantly Precise Compositions of Materials!
Total Materia Horizon contains chemical compositions of hundreds of thousands materials and substances, as well as their mechanical and physical properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.