Corrosion Resistance of Ferritic Stainless Steels
Abstract
Ferritic stainless steels have certain useful corrosion properties, such as resistance to chloride stress-corrosion cracking, corrosion in oxidizing aqueous media, oxidation at high temperatures and pitting and crevice corrosion in chloride media. These steels contain above approximately 13% Cr and precipitate a prime phase in 350°C to 540°C range, and the maximum effect is at about 470°C. Because precipitation hardening lowers temperature ductility, it must be taken into account in both processing and usage of ferritic stainless steels, especially those with higher chromium content.
Ferritic stainless steels have certain useful corrosion properties, such as resistance to chloride stress-corrosion cracking, corrosion in oxidizing aqueous media, oxidation at high temperatures and pitting and crevice corrosion in chloride media.
These steels contain above approximately 13% Cr and precipitate a prime phase in 350oC to 540oC range, and the maximum effect is at about 470oC. Because precipitation hardening lowers temperature ductility, it must be taken into account in both processing and usage of ferritic stainless steels, especially those with higher chromium content.
Structures of these steels are kept completely ferritic at room and high temperature by adding titanium or columbium, or by melting to very low levels of carbon and nitrogen, or both. Such microstructures provide ductility and corrosion resistance in weldments. Molybdenum improves pitting corrosion resistance, while silicon and aluminum increase resistance to high temperature oxidation.
The newer ferritic steels with high content of chromium have become possible through vacuum and argon-oxygen decarburization, electron-beam melting, and large-volume vacuum induction melting. The representatives of this group include ASTM designations 409 and 439.
Type 409 with 12% Cr is relatively low-cost and has good formability and weldability. Recommended thickness is limited to approximately 3,8 mm maximum if ductile-to-brittle transition temperature (DBTT) at room temperature or lower is needed (Figure 1). Its atmospheric corrosion resistance is adequate for functional uses, so applications of this type of steel include automobile exhaust equipment, radiator tanks, catalytic reactors, containerization and dry fertilizer trunks.
Type 439 with 18-20% Cr resists chloride stress-corrosion cracking. Resistance to general and pitting corrosion is approximately equivalent to that of austenitic types 304 and 316. This grade is suitable for equipment exposed to the aqueous chloride environments, heat transfer applications, condenser tubing for fresh water power plants, food-handling uses and water tubing for domestic and industrial buildings. Sheet thickness cannot exceed approximately 3,2 mm if DBTT (Figure 1) at room temperature or lower is needed.
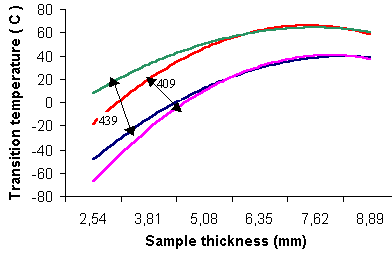 |
| Figure 1. Ductile-to-brittle transition temperatures (DBTT) for ferritic stainless steel rise with section thickness. Bands for 409 and 439 indicate data scatter |
Resistance to stress-corrosion cracking is the most obvious advantage of the ferritic stainless steels. Ferritic steels resist chloride and caustic stress corrosion cracking very well. Nickel and copper residuals lower resistance of these steels to stress corosion.
Susceptibility of the ferritic steels to intergranular corrosion is due to chromium depletion, caused by precipitation of chromium carbides and nitrides at grain boundaries. Because of the lower solubility for carbon and nitrogen and higher diffusion rates in ferrite, the synthesized zones of welds in ferritic steels are in the weld and adjacent to the weld. To eliminate the intergranular corrosion, it is necessary either to reduce carbon to very low levels, or to add titanium and columbium to tie up the carbon and nitrogen.
Pitting, an insidious localized type of corrosion occurring in halide media, can put complete installations out of operation in relatively short time. Resistance to this type of corrosion depends on chloride concentration, exposure time, temperature and oxygen content. In general, resistance to pitting increases with chromium content. Molybdenum also plays an important role and it is equivalent to several percentages of chromium.
General corrosion resistance: The atmospheric corrosion resistance of the ferritic steels is excellent. These steels have good corrosion resistance in strongly oxidizing environments, such as nitric acid. In organic acids, all ferritic steels are superior to austenitic, but in reducing media general corrosion resistance of ferritic steels is worst than austenitic.
High-chromium ferritic stainless steels
High-chromium ferritic stainless steels - such as types 442 and 446 - have excellent resistance to corrosion and to oxidation in many industrial environments. These alloys are included in ASTM specifications A176-74 (Chromium stainless flat products), A 511 (Seamless stainless steel mechanical tubing), A268-74 (Ferritic stainless steel tubing for general service) and also in ASME code and AISI and SAE specifications.High-chromium ferritic steels have 18-30% Cr and low content of carbon and nitrogen. Titanium in these alloys prevents intergranular chromium-carbide and nitride precipitation during welding or processing. Because of the ferritic structure and controlled composition, the alloys exhibit good resistance to general, intergranular and pitting corrosion, and stress corrosion cracking. Similar to other high chromium stainless steels, types 442 and 446 have excellent oxidation resistance at elevated temperatures. They also have high thermal conductivity, higher yield strength than austenitic stainless steels, and lower tensile ductility.
The excellent resistance to chlorides, organic acids and chloride stress-corrosion indicates that these alloys should be suitable for a wide range of applications in which conventional stainless steels or other materials are either inadequate or uneconomical. High-chromium ferritic stainless steels are useful in heat exchanger tubing, feed-water tubing and in equipment that operate with chloride-bearing or brackish cooling waters.
Available in sheet, strip, tubing and welding wire, alloys are finding substantial application in replacing brass and cupronickel, corrosion-resistant high-nickel alloys, and other materials in the food processing, power, chemical, petrochemical, marine and pulp and paper industries.
Access Precise Properties of Stainless Steels Now!
Total Materia Horizon contains property information for 120,000+ stainless steels: composition, mechanical and physical properties, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.