Tungsten Carbide Metals: Part One
Abstract
Tungsten Carbide is a remarkable material renowned for its use in some of the most demanding industrial applications. Classified as a refractory carbide, it exhibits exceptional mechanical and thermal properties, including a melting point exceeding 1800°C and outstanding hardness. Its high wear resistance makes it indispensable for extending part life in production processes, with applications ranging from tooling to extreme-environment electronics substrates.
Introduction to Tungsten Carbide
Tungsten Carbide combines tungsten, the metal with the highest melting temperature among metallic elements, with carbon. This combination forms a refractory carbide with superior properties such as extreme hardness (2000–2700 HV) and excellent chemical resistance. Tungsten Carbide exists in two primary phases, WC and W2C, each with distinct characteristics and uses.
Tungsten Carbide Phases and Properties
Tungsten Carbide powders are typically found as monocristalline WC with a carbon content of approximately 6.1%, or as eutectic WC/W2C with about 4% carbon, as illustrated in the tungsten-carbon phase diagram (Figure 1).
Three main stoichiometries include:
- W2C: Exhibits a wide homogeneity range (25.5–34 at.% C at 2715°C) and forms from a eutectoidal reaction between elemental W and δ-WC at 1250°C. It melts congruently with the W solid solution at 1715°5°C.
- γ-WC1-x: Results from a eutectoidal reaction between β and δ phases at 2535°C and melts at approximately 2785°C. This phase can be produced at room temperature through rapid cooling.
- δ-WC: The only stable binary phase at room temperature, with no solid solubility up to 2384°C, though it becomes carbon deficient at higher temperatures.
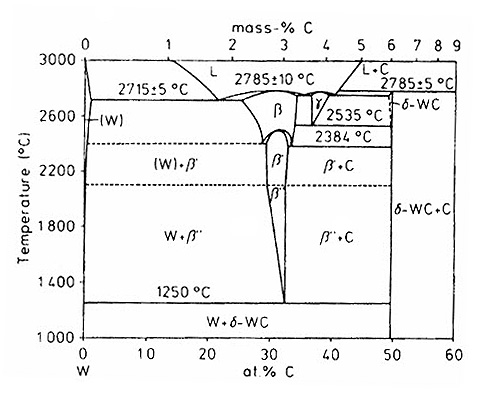
Figure 1: W-C Phase Diagram
This diagram illustrates the tungsten-rich section of the binary W-C equilibrium, detailing the various phases and their formation conditions.
Nominal Properties of Tungsten and Tungsten Carbides
Tungsten Carbide's remarkable attributes make it ideal for demanding applications. Key properties are summarized below in Table 1.
Table 1. Tungsten and Tungsten Carbide Properties
| Type | Melting Point | Density |
| W | 3410°C | 19.3 [g/cm³] |
| WC | 2870°C | 17.2 [g/cm³] |
| W₂C | 2730°C | 15.8 [g/cm³] |
- High Hardness: 2000–2700 HV
- Melting Point: Exceeds 1800°C
- Chemical Resistance: Good
Applications of Tungsten Carbide
Tungsten Carbide's exceptional properties make it invaluable in various industries:
- Tooling and Mechanical Processing: Used in hard metal tools, often sintered with a metallic binder like cobalt or nickel for enhanced performance.
- Mining Industry: Coats drilling and digging parts to increase durability.
- Agriculture: Applied on plough shares, tillage equipment, and cutters.
- Steel Industry: Serves as coatings for guide rollers.
- Oil Industry: Protects components in high-wear applications.
Surface Coatings and Metal Matrix Composites
Tungsten Carbide is commonly used in surface coatings to enhance wear resistance. Cemented Carbides, formed by sintering tungsten carbide with metallic binders, require cutting and grinding tools of even harder materials like Cubic Boron Nitride (CBN) or diamond discs.


Figure 2: Applications of Tungsten Carbide Coatings
This figure depicts various uses, including coated plough shares, digging equipment, and scrapers, showcasing the versatility and durability of Tungsten Carbide.
Read more
Find Instantly Precise Properties of Refractory Metals!
Total Materia Horizon contains property information for thousands of refractory alloys: composition, mechanical and physical properties, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.