Aluminum-Lithium Alloys: Part Three
Abstract
The advantages of aluminum-lithium alloys are numerous and include improved weldability, reduced density, and an improved modulus of elasticity and precipitation hardening.
When considering the stringent requirements of the aerospace industry however, certain properties still require improvement including overall strength and fracture toughness.
Table 1 lists the chemical composition of various Al–Li alloys that have been industrially produced over the past thirty years. The forerunner Al–Li alloy to be developed and used was the Al–Mg–Li–Zr alloy 1420. In this alloy, the beneficial effect of 50 wt.% Mg addition on solid solution strengthening and on improved weldability was combined with the advantages of reduced density, enhanced modulus and precipitation hardening provided by 2 wt.% Li addition. A trace addition of almost 01 wt.% Zr was made to control recrystallization and grain growth. Alloy 1420 was used in the MIG-29 aircraft fuselage in the form of welded structures in the early 1980’s and subsequently applied to passenger aircrafts.
However, the strength and fracture toughness of alloy 1420 were not adequate to meet the demands of modern aircraft, even in terms of specific properties. The reasons for poor fracture toughness, apart from those attributable to impurities, were related to shearing of the main strengthening phase δ’ (Al3Li) which led to planar slip – an aspect discussed in detail in the next section. Further research was therefore, directed towards compositions that promised additional non-shearable precipitates that would reduce the tendency for planar slip and lead to further hardening of the alloys.
As a result of the above, alloys such as AA 8090, AA 2090, AA 2091, 1441, 1460 were developed around the world. In addition to the δ’ precipitate, these alloys contained either one or both of the T1(Al2CuLi) and S (Al2CuMg) precipitate, that served to improve fracture toughness of these alloys.
Al–Li alloys have undergone several more changes in composition in the last ten years. First, the Li content of some of the alloys was reduced to < 2 wt.%. One such alloy, Weldalite-049 (AA2095), was developed specially for welded fuel tank applications. This alloy contained higher Cu compared to other Al–Li based alloys and derived its strength mainly from the T1 phase. In this alloy, Li helped improve the specific modulus.
The other alloy with lower Li content was the Russian sheet alloy 1441. Reduction in Li (< 2 wt.%) and Zr (=0.10 wt.%) content, combined with controlled rolling, allowed sheets to have fine equiaxed grain morphology, controlled texture and improved in-plane isotropy of properties.
More recent compositional changes have mainly focused on trace element additions like Ag, Ce, Y and Sc. Of these, Sc addition has been the most successful in microstructure refinement and improved microstructural stability at high temperatures. Some of the Sc-modified variants of alloy 1420 are produced commercially in Russia and used widely for welded applications.
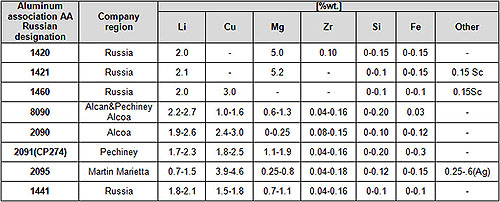
Table 1: Chemical composition of various industrial Al-Li alloys
The objective of Novitović’s O. et al paper was to study the precipitation mechanism of Al-Li alloy (8091) at strengthening maximum (peak hardness) applying quantitative microstructural analysis. Also, the identification of precipitates was one of the aims of this paper. Al-Li alloy (8091) with chemical composition 2.40 Cu, 2.0 Mg, 0.70 Zr, 1.15 Li (mass %) was solution treated at 540°C for 30 min, water-quenched and continuously heated in oil at different heating rates (0.05 and 0.1°C/min), from room temperature to 190°C.
According to previous experiments it was well documented that by aging of the supersaturated Al-Li (8091) solid solution, the maximum value of hardness (peak hardness) was achieved at this temperature. Reaching 190°C at pre-determined continuous heating, samples were water-quenched then cooled in liquid nitrogen in order in to prevent natural aging. Holding time at 190°C was 700 and 50min for samples heated at rates of 0.05 and 0.1oC/min, respectively.
Samples with dimensions 10x10x1mm were thinned by emery paper until the foil with a thickness of between 0.1 and 0.08mm was obtained. The disks with a diameter of 3mm punched from this foil were electrochemically polished. The polishing was performed at room temperature in the „Jet-pol“ apparatus using a mixture of 10% HClO4 and 90% ethanol, under the following conditions: voltage – 14V, current – 20mA, time of polishing – approximatelly 60s. TEM „JEM 100C–JEOL“used for microstructural examination was operated at 100kV.
A Vickers hardness with load of 10 kgf was applied to measure hardness of heat treated samples.
Quantitative microstructural analysis was carried out by a semi-automatic device for picture analysis with automatic data processing, using microphotographs produced by light and TEM. Structural parameters which best define processes and features were measured. They are primarily the parameters which characterize precipitated particles, i.e. the particle size, the volume fraction and the numerical density, as well as the distance between the particles.
Conclusion
Precipitation in the Al-Li (8091) alloy was studied during heating (0.05 and 0.1°C/min) from room temperature to peak hardness (190°C). Size, shape, density, distribution and other characteristics of precipitates were determined applying the method of quantitative metallographic analysis.
Precipitate identification performed by transmission electron microscopy (TEM) revealed the presence of strengthening phases formed during heating to 190°C: δ’ (Al3Li), S (Al2CuMg), and β’ (Al3Zr).
Very fine δ’ particles rather elliptic than spherical in shape together with lath shaped S phase may be seen in samples heated with both heating rates. Also, dislocation loops of various diameters are present in Al-matrix. The size of the δ’ particles are approximately 26% larger during the faster cooling rate.
With the lower heating rate, a higher hardness was obtained than in the case when the higher heating rate was applied. Higher values of hardness may be ascribed to the smaller size of δ' particles and their homogeneous and dense distribution in the Al matrix.
Access Precise Properties of Aluminum Alloys Now!
Total Materia Horizon contains property information for 30,000+ alumiums: composition, mechanical, physical and electrical properties, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.