High Strength Copper-Titanium Alloys: Part One
Abstract
Copper-titanium (Cu-Ti) alloys are emerging as ultra-high strength conductive materials that effectively replace conventional copper-beryllium (Cu-Be) alloys in applications such as conductive springs and interconnections. This transition has been accelerated by recognition of serious health hazards associated with beryllium-based production processes. While Cu-Ti alloys demonstrate comparable mechanical properties to Cu-Be alloys, with superior high-temperature strength and stress relaxation behavior, their electrical conductivity is somewhat lower. The age-hardening mechanisms, microstructural evolution, and phase transformations in Cu-Ti alloys containing 1-5 wt.% Ti are examined, with particular focus on the formation of the metastable D1a precipitate phase that contributes to their remarkable strength properties.
Introduction to Cu-Ti Alloys
The age hardening of copper-titanium (Cu-Ti) alloys containing approximately 1-5 wt.% Ti (1-6 at.% Ti) has been known since the 1930s. These alloys exhibit mechanical and physical properties comparable to the widely used copper-beryllium (Cu-Be) alloys, with better high-temperature strength and superior stress relaxation behavior. The electrical conductivity of the aged alloys, however, falls somewhat below that of the Cu-Be alloys.
Cu-Ti alloys are now receiving significant attention as ultra-high strength conductive materials for applications such as conductive springs and interconnections, essentially displacing the conventional Cu-Be alloy series. This move away from Cu-Be has been catalyzed largely by the full recognition of serious health hazards associated with beryllium-based metallurgy in production. It is likely that precipitation-hardened Cu-Ti alloys will rise to prominence as a commercial alternative in numerous applications over the next decade.
Manufacturing Process and Structural Characteristics
Many electrically conductive springs of thin plate type are manufactured from age-hardened copper-titanium alloy due to its excellent mechanical strength and electrical conductivity. These spring sheets are typically produced through a process that includes preparing a copper-titanium melt, casting, hot working, alternating annealing and cold working to achieve final shape, solution heat treatment, and age-hardening after cold working when required. The solution heat-treated structure, however, has an average crystal grain size of at least 40 microns and sometimes up to 100 microns.
While the age-hardened copper-titanium alloy was developed as an inexpensive substitute for copper-beryllium alloy (as disclosed in U.S. Pat. No. 4,425,168 issued to Goldstein et al. on January 10, 1984), conventional age-hardened copper-titanium alloys remain unsatisfactory in several aspects. Improvements are needed regarding mechanical properties such as formability, fatigue strength, elongation, and yield strength. Additionally, these alloys exhibit the drawback of having different properties in the rolling direction versus the perpendicular direction.
Phase Diagrams and Microstructural Evolution
Early phase diagrams of the Cu-Ti system showed the terminal FCC Cu-rich solid solution (α) to be in equilibrium with a Cu3Ti phase characterized by the Pnmm space group. However, subsequent research identified an Au4Zr-type structure, with the orthorhombic Cu4Ti exhibiting the Pnma space group.
Research in the early 1970s by Hakkarainen, Laughlin, and Cahn revealed that a metastable coherent precipitate phase with the tetragonal D1a structure (Ni4Mo-type; I 4/m) having the composition Cu4Ti forms during aging below approximately 600–700°C. This important finding was further verified in a comprehensive electron microscopy study of microstructural behavior in alloys containing 1–4 wt.% Ti conducted by Datta and Soffa. The resulting metastable, finely dispersed two-phase mixtures of α'+D1a that develop during aging produce highly aligned and quasi-periodic microstructures, often called modulated structures.
During prolonged aging at low or moderate temperatures, a coarse lamellar microconstituent composed of the equilibrium phase and terminal solid solution (α) nucleates and grows, consuming the fine-scale dispersion of coherent/semicoherent D1a particles.
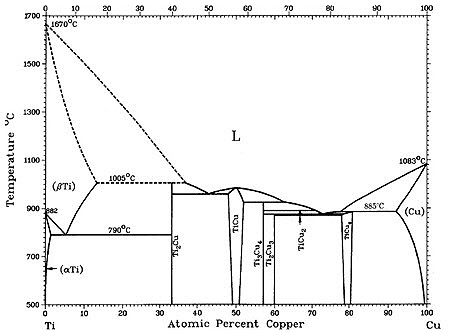
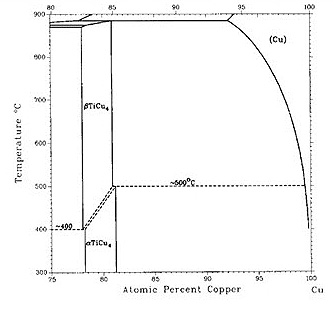
Figure 1: Figure 1(a-b) shows recent versions of the equilibrium diagram and the measured metastable solvus of the D1a precipitate phase. The portion of the Cu–Ti equilibrium diagram in Figure 1b indicates a polymorphic transformation of the Cu4Ti phase, suggesting that the tetragonal D1a phase is the stable phase below approximately 500°C, while the orthorhombic Au4Zr-type structure is the equilibrium high-temperature phase.
The existence of the D1a structure as the stable low-temperature phase is not surprising since the D1a structure is a ground state for FCC-based alloys. The orthorhombic Pnma structure (Au4Zr-type) is presumably stabilized at higher temperatures by entropic effects.
Scientific Interest and Commercial Implications
Fundamental interest in Cu-Ti age-hardening alloys emerged in the 1960s and 1970s when these alloys were recognized as prototypical binary "sideband alloys" potentially exhibiting spinodal decomposition. Their strong age-hardening response received renewed attention when these precipitation-hardening alloys demonstrated extraordinary plastic flow behavior, including profuse deformation twinning.
The occurrence of spinodal decomposition during the early stages of precipitation, resulting in ordered phase formation, prompted new thinking regarding the interplay of clustering and ordering tendencies in solid solutions, similar to theories articulated by Allen, Cahn, and Ino. As commercial use of age-hardened Cu-Ti alloys expands, the nature of the cellular reaction or discontinuous precipitation will undoubtedly become a major focal point, since the appearance of cellular microconstituents leads to detrimental effects on mechanical properties. Additionally, the influence of plastic deformation on the aging response will be of paramount importance in the thermomechanical processing of these materials in production.
Numerous experimental methods have been applied to elucidate the basic mechanisms controlling the formation of characteristic periodic and aligned two-phase microstructures that arise during aging. These methods include X-ray and neutron diffraction, electron microscopy and diffraction, and atom probe field-ion microscopy (APFIM). The early stages of decomposition and the origin of the "sideband state" will be discussed along with the evolution of microstructures during prolonged aging.
Decomposition Mechanisms
The decomposition of supersaturated Cu-Ti alloys containing 1-6 atomic % titanium involves a complex interplay of clustering and ordering effects in solid solutions, as well as a subtle synergy between short-range order (SRO) and long-range order (LRO). Additionally, these supersaturated alloys exhibit well-known "sideband" phenomena in diffraction patterns during the early stages of decomposition.
As previously noted, the precipitate phase of particular interest in the age-hardening response of these high-strength alloys exhibits the D1a superstructure (Ni4Mo-type; I 4/m) and is therefore the focus of this discussion. A generalized Bragg-Williams description of the thermodynamics of the LRO Cu4Ti/D1a structure requires fourth-nearest neighbors to stabilize the structure as a non-degenerated ground state for FCC-based superstructures. However, third-nearest-neighbor interactions will lead to the emergence of SRO and LRO structures from a disordered solid solution at low temperatures.
The A4B superstructure is favored in systems where the first nearest-neighbor interchange potential V(1) and second nearest-neighbor interchange potential V(2) have the same sign when considering just the first and second nearest-neighbors in the structural energies of various FCC-based superstructures.
Access Precise Properties of Copper Alloys Now!
Total Materia Horizon contains property information for 30,000+ copper alloys: composition, mechanical, physical and electrical properties, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.