Nickel-Based Superalloys: Part One
Abstract
Superalloys are metallic materials for service at high temperatures, so one of their most important properties is high temperature creep resistance. Other crucial material properties are fatigue life, phase stability, as well as oxidation and corrosion resistance.
Superalloys develop high temperature strength through solid solution strengthening. Oxidation and corrosion resistance is provided by the formation of a protective oxide layer which encapsulates the material, and thus protecting the rest of the component.
A superalloy, or a high-performance alloy, is an alloy that exhibits excellent mechanical strength and creep resistance at high temperatures, good surface stability, and corrosion and oxidation resistance. A superalloy's base alloying element is usually nickel, cobalt, or nickel-iron. Superalloy development has relied heavily on both chemical and process innovations and has been driven primarily by the aerospace and power industries.
Superalloys are metallic materials for service at high temperatures, particularly in the hot zones of gas turbines. Such materials allow the turbine to operate more efficiently by withstanding higher temperatures. Turbine Inlet Temperature (TIT), which is a direct indicator of the efficiency of a gas turbine engine, depends on the temperature capability of first stage high pressure turbine blade made of Ni base superalloys exclusively.
One of the most important superalloy properties is high temperature creep resistance. Other crucial material properties are fatigue life, phase stability, as well as oxidation and corrosion resistance.
Superalloys develop high temperature strength through solid solution strengthening. Oxidation and corrosion resistance is provided by the formation of a protective oxide layer which is formed when the metal is exposed to oxygen and encapsulates the material, and thus protecting the rest of the component. Oxidation or corrosion resistance is provided by elements such as aluminum and chromium. By far the most important strengthening mechanism is through the formation of secondary phase precipitates such as gamma prime and carbides through precipitation strengthening.
As mentioned earlier, creep and oxidation resistance are the prime design criteria. Superalloys can be based on iron, cobalt or nickel, the latter being best suited for aeroengine applications. The essential solutes in nickel based superalloys are aluminum and/or titanium, with a total concentration which is typically less than 10 atomic percent. This generates a two-phase equilibrium microstructure, consisting of gamma (γ) and gamma-prime (γ'). It is the γ' which is largely responsible for the elevated-temperature strength of the material and its incredible resistance to creep deformation. The amount of γ' depends on the chemical composition and temperature, as illustrated in the ternary phase diagrams below.
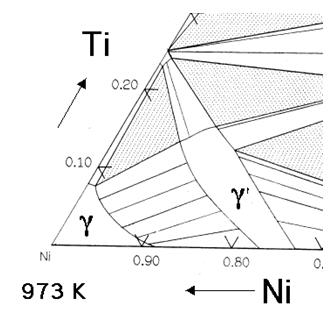
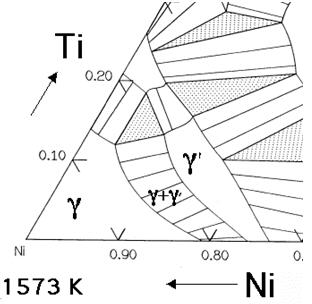
Figure 1: The Ni-Al-Ti ternary phase diagrams show the γ and γ' phase field.
The Ni-Al-Ti ternary phase diagrams show the γ and γ' phase field (Fig.1). For a given chemical composition, the fraction of γ' decreases as the temperature is increased. This phenomenon is used in order to dissolve the γ' at a sufficiently high temperature (a solution treatment) followed by ageing at a lower temperature in order to generate a uniform and fine dispersion of strengthening precipitates.
The γ-phase (Fig.2a) is a solid solution with a cubic-F lattice and a random distribution of the different species of atoms. Cubic-F is short for face-centered cubic. By contrast, γ' has a cubic-P (primitive cubic) lattice (Fig. 2b) in which the nickel atoms are at the face-centers and the aluminum or titanium atoms at the cube corners. This atomic arrangement has the chemical formula Ni3Al, Ni3Ti or Ni3 (Al, Ti). However, as can be seen from the (γ+γ')/γ' phase boundary on the ternary sections of the Ni, Al, Ti phase diagram, the phase is not strictly stoichiometric. There may exist an excess of vacancies on one of the sublattices which leads to deviations from stoichiometry; alternatively, some of the nickel atoms might occupy the Al sites and vice-versa. In addition to aluminum and titanium, niobium, hafnium and tantalum partition preferentially into γ'.
The γ phase forms the matrix in which the γ' precipitates. Since both the phases have a cubic lattice with similar lattice parameters, the γ' precipitates in a cube-cube orientation relationship with the γ (Fig. 2a and 2b). This means that its cell edges are exactly parallel to corresponding edges of the γ phase. Furthermore, because their lattice parameters are similar, the γ' is coherent with the γ when the precipitate size is small. Dislocations in the γ nevertheless find it difficult to penetrate γ', partly because the γ' is an atomically ordered phase. The order interferes with dislocation motion and hence strengthens the alloy.
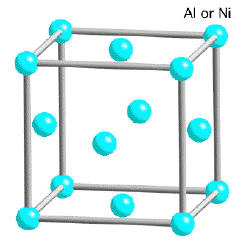
Figure 2a: Crystal structure of γ.
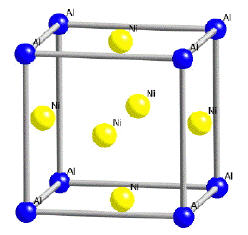
Figure 2b: Crystal structure of γ'.
The small misfit between the γ and γ' lattices is important for two reasons. Firstly, when combined with the cube-cube orientation relationship, it ensures a low γ/γ' interfacial energy. The ordinary mechanism of precipitate coarsening is driven entirely by the minimization of total interfacial energy. A coherent or semi-coherent interface therefore makes the microstructure stable, a property which is useful for elevated temperature applications.
The magnitude and sign of the misfit also influences the development of microstructure under the influence of a stress at elevated temperatures. The misfit is said to be positive when the γ' has a larger lattice parameter than γ. The misfit can be controlled by altering the chemical composition, particularly the aluminum to titanium ratio. A negative misfit stimulates the formation of rafts of γ', essentially layers of the phase in a direction normal to the applied stress. This can help reduce the creep rate if the mechanism involves the climb of dislocations across the precipitate rafts.
Read more
Access Precise Properties of Nickel and Superalloys Now!
Total Materia Horizon contains property information for 20,000+ nickel alloys: composition, mechanical and physical properties on various temperatures, corrosion resistance, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.