Metal Vacuum Distillation Process: Part One
Abstract
Vacuum metallurgy techniques offer significant advantages in metal processing, including reduced energy consumption, lower pollution levels, and streamlined processing workflows. Vacuum distillation technology enables the separation and purification of metals with different boiling points by reducing atmospheric pressure, thereby lowering the required temperatures for metal volatilization. This process is particularly effective for removing impurities such as mercury, cadmium, zinc, antimony, lead, and tin from various alloys. The technology provides flexibility in producing either liquid metal or nanoscale powder through controlled condensation rates. Research demonstrates that vacuum distillation can effectively remove copper, tin, silver, zinc, arsenic, and antimony from crude lead based on vapor pressure differences, though bismuth separation remains challenging due to similar thermodynamic properties.
Introduction to Vacuum Distillation Metallurgy
Vacuum metallurgy has emerged as a transformative technology in modern metal processing, offering substantial improvements over traditional refining methods. This advanced approach addresses many limitations of conventional metallurgical processes while providing enhanced environmental benefits and operational efficiency. The vacuum distillation process represents a specialized metallurgical technique that leverages reduced atmospheric pressure to significantly decrease the boiling points of metals within alloys, enabling selective separation and purification.
Fundamental Principles of Metal Vacuum Distillation
The core principle underlying vacuum distillation technology relies on the distinct vapor pressure characteristics exhibited by different metals at varying temperatures. Under vacuum conditions, metals with lower boiling points can be selectively volatilized and separated from those with higher boiling points. This thermodynamic behavior forms the foundation for efficient metal separation and purification processes.
The vacuum distillation process is particularly well-suited for treating alloys containing mercury (boiling point: 357°C), cadmium (767°C), zinc (907°C), antimony (1380°C), lead (1620°C), and tin (2260°C). By controlling condensation speed and processing parameters, manufacturers can produce either liquid metal or nanoscale powder products to meet diverse client requirements.
Thermodynamic Analysis of Crude Lead Vacuum Distillation
Recent research has systematically investigated the thermodynamic performance of various components during crude lead vacuum distillation processes. This analysis provides valuable insights for developing simple, clean, and efficient methods for removing copper, tin, silver, zinc, arsenic, and antimony impurities from crude lead.
Vapor Pressure Relationships and Temperature Dependencies
The relationship between saturated vapor pressure and temperature for the main components follows the established equation:
lg p*= AT⁻¹ +Blg T+CT+D
Where p* represents the saturated vapor pressure and T denotes the temperature in Kelvin.
Table 1. The evaporation constants A, B, C, and D for different metallic components
| Component | A | B | C | D | T/K |
| Pb | -10130 | -0.985 | - | 13.280 | 600–2013 |
| Cu | -17520 | -1.210 | - | 15.330 | 1356–2840 |
| Sn | -15500 | - | - | 10.355 | 505–2473 |
| Ag | -14400 | -0.850 | - | 13.825 | 1233–2468 |
| Zn | -6620 | -1.255 | - | 14.465 | 692–1773 |
| As | -6160 | - | - | 11.945 | 873–1773 |
| Sb | -6500 | - | - | 8.495 | 904–1860 |
| Bi | -10400 | -1.260 | - | 14.470 | 544–1837 |
Analysis of Vapor Pressure Data
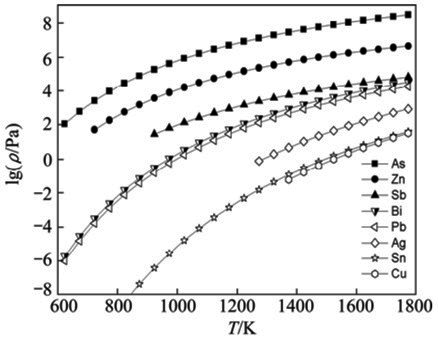
Figure 1: Calculated saturated vapor pressure relationships
The calculated saturated vapor pressure data reveals significant differences in volatilization behavior among various metallic components. Arsenic and zinc demonstrate substantially higher saturated vapor pressures compared to lead across the temperature range of 873-1073 K. Notably, arsenic begins sublimation at 823 K, indicating that both arsenic and zinc readily volatilize into the vapor phase and can be completely removed from crude lead during processing.
Antimony exhibits relatively high saturated vapor pressure compared to lead, enabling partial removal at appropriately controlled temperatures. Conversely, copper, tin, and silver display much lower saturated vapor pressures than lead within the 1273-1523 K temperature range, indicating these elements resist volatilization and tend to concentrate in the residual phase.
Bismuth presents a unique challenge, as its saturated vapor pressure closely matches that of lead, making separation through vacuum distillation thermodynamically unfavorable.
Process Optimization and Operational Guidelines
Based on comprehensive thermodynamic analysis, several key conclusions emerge regarding vacuum distillation optimization for crude lead processing:
Selective Impurity Removal Strategies
The vacuum distillation process enables effective removal of copper, tin, silver, zinc, arsenic, and antimony impurities from crude lead through thermodynamically favorable conditions. However, bismuth removal remains challenging due to similar vapor pressure characteristics with lead.
Temperature-Controlled Processing Approach
Optimal vacuum distillation requires a two-stage temperature approach. Initial processing at lower temperatures (923-1023 K) effectively removes zinc, arsenic, and antimony through selective volatilization. Subsequently, lead distillation from the remaining liquid occurs at elevated temperatures (1323-1423 K), while copper, tin, and silver concentrate in the residual liquid phase.
Thermodynamic Calculations for Process Design
Comprehensive thermodynamic calculations prove essential for selecting appropriate operational conditions and achieving reliable results in vacuum distillation refining processes. These calculations enable precise control of separation efficiency and product quality while optimizing energy consumption and processing time.
Environmental and Economic Advantages
Vacuum distillation technology offers significant environmental benefits compared to traditional metallurgical processes. The reduced energy consumption requirements, combined with lower pollution levels and streamlined processing workflows, contribute to more sustainable metal production practices. Additionally, the technology's flexibility in producing various product forms enhances its commercial viability across diverse market applications.
Conclusion
Vacuum distillation represents a highly effective metallurgical technology for metal separation and purification, particularly in crude lead processing applications. The process leverages fundamental thermodynamic principles to achieve selective metal separation while offering environmental and economic advantages over conventional methods. Through careful temperature control and optimization of processing parameters, vacuum distillation enables efficient removal of multiple impurities while maintaining product quality and operational efficiency.
Find Instantly Thousands of Heat Treatment Diagrams!
Total Materia Horizon contains heat treatment details for hundreds of thousands of materials, hardenability diagrams, hardness tempering, TTT and CCT diagrams, and much more.

Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.