Graphene Materials Applications
Abstract
Graphene is a single layer of graphite which is commonly known as an allotrope of carbon, and was first isolated in 2004 using simple, household scotch tape.
One hundred times stronger than steel along with many other superior characteristics, graphene is beginning to be used in a range of application such as; solar cells, transistors and television screens.
Today, a new material has the potential to alter the future. Dubbed a “supermaterial,” graphene has researchers the world over scrambling to better understand it. Graphene’s long list of miraculous traits makes it seem almost magical, but it could have very real and drastic implications for the future of physics and engineering.
The history of graphene: A roll of tape, and a dream
Graphite has been a known quantity for a long time (humans have been using it since the Neolithic era). Its atomic structure is well documented, and for a long time, scientists pondered whether single layers of graphite could be isolated. Until recently, however, graphene was merely a theory, as scientists were unsure if it would ever be possible to slice graphite down to a single, atom-thin sheet. The first isolated sample of graphene was discovered in 2004 by Andre Geim and Konstantin Novoselov at the University of Manchester. One might expect that they isolated the fabled substance using some massive, expensive piece of machinery, but the tool they used was amusingly simple: A roll of scotch tape.
What exactly is graphene?
The simplest way to describe graphene is that it is a single, thin layer of graphite — the soft, flaky material used in pencil lead. Graphite is an allotrope of the element carbon, meaning it possesses the same atoms but they’re arranged in a different way, giving the material different properties. For example, both diamond and graphite are forms of carbon, yet they have wildly different natures. Diamonds are incredibly strong, while graphite is brittle. Graphene’s atoms are arranged in a hexagonal arrangement.
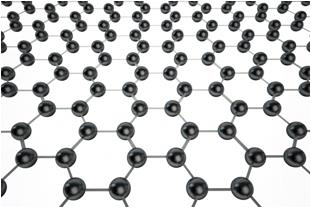
Figure 1: Graphene’s atoms
Interestingly, when graphene is isolated from graphite it takes on some miraculous properties. It is a mere one-atom thick, the first two-dimensional material ever discovered. Despite this, graphene is also one of the strongest materials in the known universe. With a tensile strength of 130 GPa (gigapascals), it is more than 100 times stronger than steel.
Graphene’s incredible strength despite being so thin is already enough to make it amazing, however, its unique properties do not end there. It is also flexible, transparent, highly conductive, and seemingly impermeable to most gases and liquids. It almost seems as though there is no area in which graphene does not excel.
What can it be used for?
The use of graphene in everyday life is not far off, due in part to existing research into carbon nanotubes — the rolled, cylindrical version of graphene.
Some of the biggest emerging applications are:
• Solar cells: Solar cells rely on semiconductors to absorb sunlight. Semiconductors are made of an element like silicon and have two layers of electrons. At one layer, the electrons are calm and stay by the semiconductor’s side. At the other layer, the electrons can move about freely, forming a flow of electricity. Solar cells work by transferring the energy from light particles to the calm electrons, which become excited and jump to the free-flowing layer, creating more electricity. Graphene’s layers of electrons actually overlap, meaning less light energy is needed to get the electrons to jump between layers. In the future, that property could give rise to very efficient solar cells. Using graphene would also allow cells that are hundreds of thousands of times thinner and lighter than those that rely on silicon.
• Transistors: Computer chips rely on billions of transistors to control the flow of electricity in their circuits. Research has mostly focused on making chips more powerful by packing in more transistors, and graphene could certainly give rise to the thinnest transistors yet. But transistors can also be made more powerful by speeding the flow of electrons — the particles that make up electricity. As science approaches the limit for how small transistors can be, graphene could push the limit back by both moving electrons faster and reducing their size to a few atoms or less.
• Transparent screens: Devices such as plasma TVs and phones are commonly coated with a material called indium tin oxide. Manufacturers are actively seeking alternatives that could cut costs and provide better conductivity, flexibility and transparency. Graphene is an emerging option. It is non-reflective and appears very transparent. Its conductivity also qualifies it as a coating to create touchscreen devices. Because graphene is both strong and thin, it can bend without breaking, making it a good match for the bendable electronics that will soon hit the market.
Find Instantly Precise Material Properties!
Total Materia Horizon contains mechanical and physical properties for hundreds of thousands of materials, for different temperatures, conditions and heat treatments, and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.