Metal Vacuum Distillation Process: Part Two
Abstract
One of the main advantages of the Vacuum Distillation Refining is that it efficiently and effectively removes impurities such as Sb, Bi, Al, Au, Ag, Cu, Si and Fe.
Another possibility using this methodology is to refine specific scrap such as magnesium into very high purity material which can be used in highly specific industrial applications (semiconductor industry in this case).
The majority of semiconductor crystal properties prepared by tellurium and cadmium are known to have strong harmful influences by the presence of trace level concentration of residual impurities since they reduce substantially detector efficiency. Purification steps of tellurium should minimize not only metallic, but also gaseous and gas-forming impurities.
There is several purification methods established to obtain 6N and above purity tellurium starting with a gross approach followed by more refined purification steps. The non-chemical routes usually followed to purify tellurium are vacuum distillation followed by horizontal zone refining. Purification processes are normally carried out in clean areas of different classes viz. 10000, 1000, 100, etc.
In a paper by D.S.Prasad et al. the vacuum distillation is performed in vacuum (~ 2 × 10–2 torr) (~ 2.67 Pa) at temperatures ranging from 450–800°C. Impurities such as Sb, Bi, Al, Au, Ag, Cu, Si and Fe can be easily separated, whereas the removal of Se, As, Na, K, Mg and S is more difficult, due to their higher vapour pressure at that temperature. Among various types of cold fingers selected for deposition viz. stainless steel, quartz, copper and alumina, the last one is found to be most suitable.
While the oxides present in tellurium at trace level wets quartz and stick upon freezing, the stainless steel and copper contaminate the deposit with impurities Cr, Ni, Mo, Fe and Pb, Cr, Ni, As, Fe, Cu, Sb, respectively in the range of 10–50 ppm. Initial experiments showed that the deposition rate increases with time and reaches its highest after the optimum time of deposition and then begins to worsen.
In conclusion the author comments that:
In purifying tellurium to 6N and above, some limitations in vacuum distillation and zone refining must be kept in mind. In addition to the process parameters in vacuum distillation such as temperature and pressure, the physical dimensions and material of construction of cold finger can act as a limitation in increasing the purity and yield. The design of quartz boat in zone refining is important to reduce the breakages. Materials handling and cleaning procedures of quartz ware with ultrapure chemicals should be checked for less than ppm level trace impurities. Hydrogen ambient gas should be better than 7N purity.
Also, an interesting alternative for recycling all types of scrap magnesium is vacuum distillation. This method aims at refining magnesium scrap into very high-purity magnesium (99.999%) to be used in the semiconductor industry. One particular application of high-purity magnesium is in blue-light laser diodes. It has been cited that this particular apparatus can improve the purity of the metal by roughly five hundred times in just one additional step. The distillation apparatus can be seen in Fig. 8. The column, crucible and vertical condenser with horizontal running high-grade graphite baffles at a number of different levels are all constructed from high-grade graphite. Steel can be used instead of high-purity graphite for the column, but that can result in slightly less pure magnesium.
The column is contained in a three-zone resistance furnace. One zone is responsible for evaporating the magnesium within the crucible, the second zone heats the condenser above the boiling point of the magnesium, and the third zone controls the temperature of the column below the boiling point of magnesium. It is desirable for the temperature to decrease with the height of the column. The baffles are responsible for lowering the speed of the vapours so that the different condensation conditions can be reached on each baffle. Each condition has the ability to condense either purified magnesium or selective impurities. The magnesium will then condense onto the baffles to produce a high-purity product. The condenser is made up of two vertical parts so that the magnesium is accessible and the baffle locations can be altered. The column is then put under vacuum.
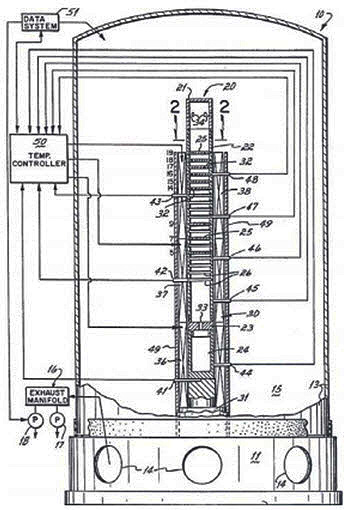
Figure 8: Patented vacuum distillation apparatus
The crucible is heated until either the boiling point of the magnesium is reached or the vapour pressure of the magnesium is higher than the vacuum pressure within the chamber. For example, a vacuum pressure of 10-7 torr (0.0000133 Pa) requires a melt temperature of 700°C. The bottom and upper parts of the condenser are maintained at temperatures of 600°C and 450°C, respectively. The two baffles at the extreme ends of the condenser control the flow of the magnesium vapours while the baffles in-between collect much of the condensed material.
Once the vapour drops below the vapour pressure of the magnesium, the metal condenses out of the gas. The higher-purity magnesium (>99.999%) is found at the ninth position and represents 55.5 wt % of the metal charge. Zinc is by far the most common impurity at roughly 6 ppm. Since zinc has a vapour pressure below that of magnesium, it will tend to condense more on the baffles located higher in the condenser. Most of the other impurities have a lower vapour pressure and will either deposit on baffles situated lower in the condenser or remain in the crucible.
In general, vacuum distillation processes are discontinuous and have a low output. The condensed fine magnesium is also highly pyrophoric rendering this process impractical for applications in the recycling industry. However, distillation under standard pressure is less complicated; it is a continuous process and has a much higher productivity, but a higher temperature is required to vaporize the magnesium. This is shown clearly in Table 1. Nonetheless, distillation is still being considered as a future alternative for refining all types of magnesium scrap.
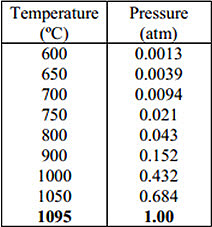
Table 1: Vapour pressure of magnesium at various temperatures
Access Thousands of Stress-Strain Diagrams Now!
Total Materia Horizon includes a unique collection of stress-strain curves of metallic and nonmetallic materials. Both true and engineering stress curves are given, for various strain rates, heat treatments and working temperatures where applicable.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.