Metal Vacuum Distillation Process: Part One
Abstract
Vacuum metallurgy techniques are widely recognized for some key advantages including short flow, lower pollution levels, and overall lower energy consumption requirements.
Another key possibility with this technology is the flexibility to be able to produce liquid metal or nanoscale powder by controlling the condensation speed.
Vacuum metallurgy has many advantages, such as short flow, low pollution and low energy consumption, and can eliminate the disadvantages of traditional refining processes. Vacuum distillation technology is a kind of metallurgy process, which can greatly decrease boiling point of low boiling point metals in alloy under vacuum, and separate and purity low boiling point metal from alloy. On the other hand, vacuum distillation has been studied and successfully used in separation of various elements from binary alloys. This technology is suitable to treat Hg 357°C, Cd 767°C, Zn °C, Sb 1380°C, Pb 1620°C, Sn 2260°C contained alloy. In order to satisfy clients’ different requirements, vacuum distillation can produce liquid metal or nanoscale powder by control of the condensation speed.
The difference in vapor pressure of each metal at different temperatures is the basic principle of crude metal vacuum distillation.
The thermodynamic performance of components of crude lead in the vacuum distillation process was investigated systemically in order to provide a simple, clean, efficient and referential way for the removal of Cu, Sn, Ag, Zn, As and Sb from crude lead.
In the paper X. Kong et al. the relationship between saturated vapor pressure of the main components and temperature is shown in Equation (1), and the evaporation constants A, B, C and D for different components are shown in Table 1.

were p* is the saturated vapor pressure; T is the temperature.
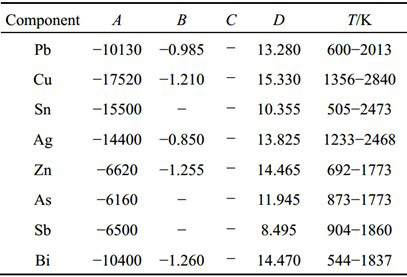
Table 1: Evaporation constants A, B, C and D for different components
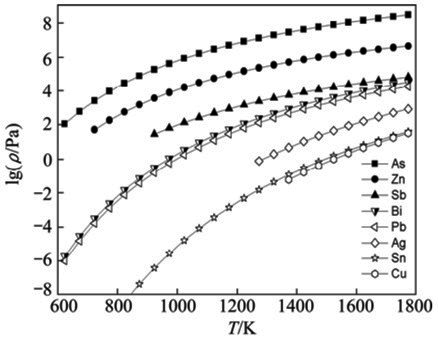
According to Equation (1) and Table 1, the saturated vapor pressure can be calculated as shown in Figure 1. Figure 1 shows that the saturated vapor pressure of As or Zn are much higher than that of Pb at 873-1073 K. At 823 K, As begins to sublimate, which indicates that As and Zn are easier to volatilize into the vapor phase and can be removed from crude lead completely.
The saturated vapor pressure of Sb is also high in comparison with Pb, which can be partially removed at an appropriate temperature. The saturated vapor pressure of Cu, Sn or Ag is much lower than that of Pb at 1273-1523 K, which shows that Cu, Sn and Ag are difficult to volatilize into a vapor phase and were concentrated in the residual phase. It also can be seen that the saturated vapor pressure of Bi is close to that of Pb, which indicates that Bi cannot be separated from lead by vacuum distillation.
Based on above mentioned paper we can conclude that:
1. The impurities of Cu, Sn, Ag, Zn, As and Sb in crude lead can be easily removed by vacuum distillation in thermodynamics, but Bi cannot be removed.
2. The vacuum distillation should be taken to obtain lead from crude lead. Zn, As and Sb are removed at lower temperature of 923-1023 K. Lead is distilled from the residual liquid containing Cu, Sn, Ag and Bi at higher temperature of 1323-1423 K, and Cu, Sn and Ag are concentrated and remain in the residual liquid.
3. The sufficient thermodynamic calculations are helpful to choose the conditions of operation and acquire reliable results in vacuum distillation refining process for crude lead.
Find Instantly Thousands of Heat Treatment Diagrams!
Total Materia Horizon contains heat treatment details for hundreds of thousands of materials, hardenability diagrams, hardness tempering, TTT and CCT diagrams, and much more.

Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.