Automotive Uses of Magnesium Alloys: Part One
Abstract
Magnesium alloys represent a significant advancement in automotive engineering due to their unique combination of light weight, high specific strength, and excellent castability. These properties make them particularly valuable for automotive and aerospace applications. The Mg-Al-based alloys, specifically the AZ and AM series, demonstrate optimal room-temperature strength and ductility while providing satisfactory salt-spray corrosion resistance and superior casting characteristics. This comprehensive review examines current applications, development methods, and future potential of magnesium alloys in automotive manufacturing.
Weight Reduction Through Magnesium Implementation
Vehicle weight reduction serves as a primary method for improving automotive fuel efficiency. While high-strength steels, aluminum, and polymers currently contribute to weight reduction, magnesium and its alloys offer additional opportunities for significant weight savings. Current automotive applications primarily involve die casting, such as housing, though potential exists for broader implementation.
Approximately 86% of a passenger car's total lifetime energy consumption relates to moving its own weight and occupants. Magnesium's inherent characteristics make it particularly attractive for automotive applications, being 36% lighter per unit volume than aluminum and 78% lighter than iron. When alloyed, magnesium demonstrates the highest strength-to-weight ratio among structural metals.
Material Evolution and Economic Considerations
Since the 1970s oil crisis, economic and legislative pressures have driven automotive weight reduction efforts. This transformation has occurred through various means, including downsizing, innovative designs, and material substitution. The shift from iron to high-strength steel (HSS), aluminum, and plastics represents significant progress, with magnesium offering further potential for weight reduction.
Sustainable Resource Management
Magnesium's abundance as the eighth most common element ensures sustainable supply. Seawater, containing 0.13% magnesium, provides virtually unlimited resources. The metal's recyclability offers additional environmental and economic benefits, though expanded U.S. production capacity would be necessary for increased automotive implementation.
Advanced Development Methodologies
The Calphad approach to computational thermochemistry has revolutionized alloy development, providing clear guidelines for element selection and reducing extensive experimentation. The current thermodynamic database encompasses 20 components (Ag-Al-C-Ca-Ce-Cu-Fe-Gd-Li-Mg-Mn-Nd-Ni-Sc-Si-Sn-Sr-Y-Zn-Zr) and 412 phases, demonstrating significant advancement in magnesium alloy development.
The exceptional combination of light weight, high specific strength, and superior castability establishes magnesium alloys as a premier engineering material for automotive and aerospace applications. Within this category, Mg-Al-based alloys, particularly the AZ and AM series, demonstrate an optimal balance of room-temperature strength and ductility, while maintaining excellent salt-spray corrosion resistance and castability characteristics.
High-Temperature Performance Requirements
Specialized automotive applications, such as engine blocks and powertrain components, demand substantial creep resistance at elevated temperatures. To meet these requirements, researchers have developed new magnesium alloys incorporating calcium and strontium additions, as well as rare earth (RE) elements. In his comprehensive research, A.A. Luo highlighted the promising high-temperature strength characteristics of magnesium-based alloys containing strontium and calcium, while noting potential manufacturing challenges related to sticking and cracking during casting. Although RE elements offer similar benefits, their usage requires careful consideration due to significant cost implications.
Performance Comparisons and Material Properties
Recent developments have yielded magnesium alloys with high-temperature properties matching those of conventional aluminum alloys. A notable example involves the comparison between an oil pan manufactured from the innovative magnesium MRI153M alloy and the traditional aluminum A380 alloy. Testing revealed comparable performance characteristics, with the magnesium alloy demonstrating superior damping properties.
Design Considerations and Material Requirements
Automotive applications demand varying material properties depending on component function. Energy absorption capacity, particularly crucial for safety components in collision scenarios, requires excellent ductility. Current alloy and process development for wrought alloys focuses on optimizing material energy absorption. However, certain components prioritize strength over ductility, necessitating diverse alloy development paths to meet specific performance requirements.
Component Integration and System Design
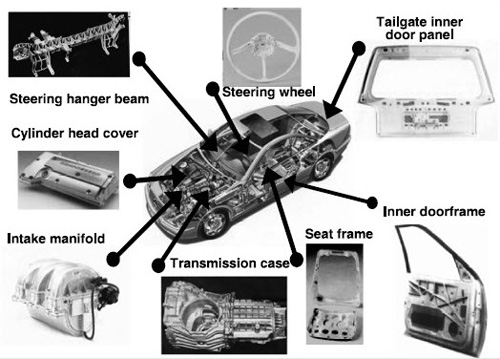
Figure 1: Automotive applications of magnesium alloys
The automotive industry relies on a select group of common materials for component manufacturing. Understanding material application requires analysis by major vehicle systems, correlating materials with specific functions and manufacturing processes. The three primary system categories - body, powertrain, and chassis - form the foundation for material selection and implementation, as demonstrated in Figure 1.
Find Instantly Precise Material Properties!
Total Materia Horizon contains physical, thermal and electrical properties for hundreds of thousands of materials, for different temperatures, and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.