Electron Beam Melting: Part Two
Abstract
The flexibility of Electron Beam Melting means that the production of both thick and thin walled components can be made which is a distinct advantage in both the automotive and aerospace industries.
In comparison to Vacuum Induction Melting, ingots produced using EBM shows carbon contamination at four to ten times lower levels than VIM demonstrating a key advantage in big reduction of contaminant levels.
Electron beam melting (EBM) was developed in the late 1990s and is used primarily in the development of fully-dense, functional, metallic components.
The EBM process is ideal for the development of metallic components that have complex surface geometries. In addition, EBM can produce both thick- and thin-walled structures. This is ideal for applications, such as components for the aerospace and automotive industries, which require low volume production of thin-walled parts that possess the strength properties of titanium and steel alloys. The ability to fabricate complex titanium components has also resulted in the use of EBM for the production of knee implants and bone plates in humans and animals. More applications of EBM are listed in Table 1.
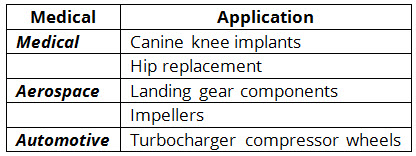
Table 1: Industrial applications of electron beam melting
Many researchers have studied the effects of high-temperature melting on the refining of pure metals. It is known that electron beam (EB) melting at high vacuum and is a very powerful technique for the refining of pure metals.
Schriempf reported that an EB zone refining process could be used to produce a final 5N-grade-purity Ru rod from commercial Ru powder that was 99.9 mass% pure. However, the purity of the 5N-grade Ru rod in that instance was estimated by means of measuring the residual resistivity ratio (RRR) value only. For this reason, a precise impurity analysis is needed to confirm the purity of refined Ru metals.
Recently, glow discharge mass spectrometry (GDMS) has been used to quantitatively determine trace elements for high-purity metals. GDMS is a direct solid-state analytical method (without any chemical process) that provides reliable quantitative concentrations below the ppb level for most elements. Therefore, the EB melting process can be used to obtain a high-purity Ru ingot for which the purity of the Ru ingot can be evaluated process using GDMS.
In the paper of Vutova K. et al a time-dependent thermal mathematical model, corresponding numerical method and computer program for simulation of heat transfer in metal ingots during EBMR are developed and presented. The mathematical model allows acquisition of important information for the practice data and dependencies, which are otherwise difficult to obtain through EBMR experimental studies (such as liquid pool geometry, energy losses, temperature distributions in metal ingots, etc.). The developed tool is employed to predict the evolution of the temperature, streams and crystallization front shape during electron beam melting and refining of different metals (hafnium, copper).
Calculated results are compared to experimental data and good correspondence is observed. In order to optimize the EBMR process, different criteria, concerning the flatness of the crystallization front shape, are proposed and discussed. Analytical optimization problems are formulated, discretized using the heat model and solved by a cluster optimization method. The developed computational modeling and optimization tools and results obtained are important and give opportunity for better understanding, studying and optimizing the EBMR process and quality improvement of the purified materials produced by this expensive modern technology.
A new alternative process to produce NiTi shape memory alloys is by electron beam melting (EBM). In the work of Otubo J. et al. the results are compared of produced ingots by two processes such as vacuum induction melting (VIM) and electron beam melting (EBM) with using a graphite crucible that contaminates the bath with carbon. The contamination by oxygen comes from residual oxygen inside the melting chamber. The results of produced ingots show that the carbon contamination is four to ten times lower for EBM compared to VIM products and that the final oxygen content is much more dependent on the starting raw materials. The purity of the final product should be very important mainly in terms of biomedical applications as the contaminations by carbon and oxygen affect the direct and reverse martensitic transformation temperatures. Table 2 shows the chemical composition of the VIM and EBM processed ingots.
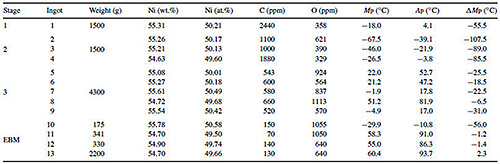
Table 2: Chemical composition of VIM and EBM processed ingots (weight-ppm)*
* Grade 1 Ti, 99.80 wt.% purity (balance); electrolytic Ni, 99.95 wt.% purity.
Read more
Access Precise Properties of Titanium Alloys Now!
Total Materia Horizon contains property information for thousands of titanium alloys: composition, mechanical and physical properties on various temperatures, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.