Extractive Metallurgy of Non-Ferrous Metals: Part Two
Abstract
Extractive metallurgy concerns the extracting, purifying and recycling of metals from an ore.
Depending on the specific extraction requirements, there is a wide range of processes and technologies in use including chemical and electrolytic reduction techniques along with mineral processing, hydrometallurgy and pyrometallurgy, available as the primary extractive metallurgical technologies.
Extractive metallurgy is the practice of extracting metal from ore, purifying it, and recycling it. Most metals found in the Earth's crust exist as oxide and sulfide minerals. These compounds must be reduced to liberate the desired metal. There are two methods of reduction: electrolytic and chemical.
Chemical reduction can be carried out in a variety of processes, including reductive smelting - the process of heating an ore along with a reducing agent (often, coke or charcoal) and purifying agents to separate the pure molten metal from the waste products. Some other processes for chemical reduction include autoclave hydrogen reduction and converting. The latter however, does not produce the pure metal, therefore requiring further treatment of its product.
Electrolytic reduction involves passing a large current through a molten metal oxide or an aqueous solution of the metal's salt. For example, aluminum is electrolyzed from bauxite dissolved in molten cryolite via the Hall-Héroult process.
Prior to reduction, it is often necessary to separate metal compounds to exclude co-reduction of different metals and contamination of the product. There is a great variety of separation processes: roasting, oxidative smelting, converting, leaching and many others.
Extractive metallurgical technologies are divided into two areas; mineral processing, hydrometallurgy and pyrometallurgy. Extractive metallurgical and mineral dressing operations are divided into:
• Ferrous metallurgy, which includes reduction of iron ore into iron, and further refinement and alloying with other metals to make steel. • Non Ferrous metallurgy, which includes all other metals. This can be further broken down into:
Precious metals - The recovery of gold and silver and the platinum group metals.
Base metals - The recovery of lead, zinc, copper, and nickel.
Light metals - The recovery of magnesium, aluminium, tin, and titanium.
Minor elements - The recovery of arsenic, selenium, bismuth, tellurium, and antimony.
Industrial minerals - Major examples include clays, sands, silicates, and heavy mineral sands.
Coal - The beneficiation and treatment of coal.
Molybdenum, a major refractory metal, is widely used as an alloying element in steels and superalloys to improve their corrosion resistance, physical properties and mechanical strength, particularly at elevated temperatures. The current worldwide production and consumption is estimated at about 130,000 tons contained molybdenum per year.
Molybdenum occurs naturally as the molybdenite (MoS2) mineral and is recovered by a flotation process as a primary concentrate at molybdenum mines and as a byproduct concentrate at copper mines. The concentrate is roasted to a technical-grade molybdic oxide (tech oxide) in multiple-hearth roasters. About 80 percent of the tech oxide is sold, in this state or after conversion to ferromolybdenum, for use as an addition to iron and steel.
The remainder is further refined to pure oxide and molybdate chemicals that are used in the manufacture of catalysts, specialty chemicals, molybdenum metal and superalloys. This paper discusses the various steps in the extractive metallurgy of molybdenum, from flotation of sulfide concentrate to reduction of pure oxide to molybdenum metal.
Many products flow from the mining and processing of ores containing molybdenite (MoS2), including:
• Chemical Mo products
• Meltstock products
• Mo metal products
Before being used, concentrate from flotation should be freed of sulfide which is done by roasting:
MoS2 + 3½ = MoO3 + 2SO2
MoS2 + 3O2 = MoO2 + 2SO2
Reaction in roasting occurs with heat evolution, and the process is carried out at 590-620 °C in Multiple heart furnaces.
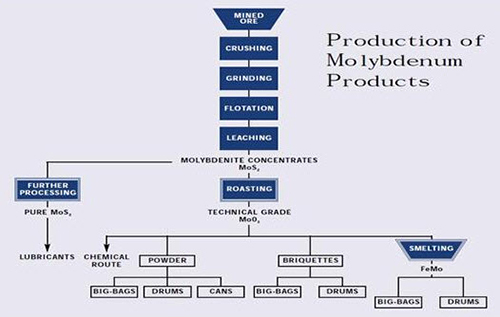
Figure 2: Production of Molybdenum Products
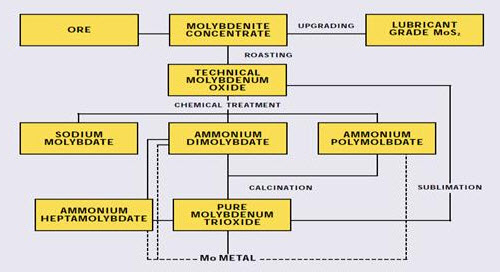
Figure 3: Production of Molybdenum chemicals
Find Instantly Precise Compositions of Materials!
Total Materia Horizon contains chemical compositions of hundreds of thousands materials and substances, as well as their mechanical and physical properties and much more.

Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.