Titanium Alloys and Their Characteristics: Part Two
Abstract
All elements which are within the range 0.85-1.15 of the atomic radius of titanium alloy substitutionally and have a significant solubility in titanium. Elements with an atomic radius less than 0.59 that of Ti occupy interstitial sites and also have substantial solubility (e.g. H, N, O, C). The ease with which solutes dissolve in titanium makes it difficult to design precipitation-hardened alloys.
The Crystal Structure of Titanium
Quenching from β phase leads to the formation of h.c.p. α' martensite. This is not particularly hard and there are increasing quantities of retained-β in the microstructure as the solute concentration increases and the MS temperature decreases.
The crystal structure of titanium at ambient temperature and pressure is close-packed hexagonal (α) with a c/a ratio of 1.587. Slip is possible on the pyramidal, prismatic and basal planes in the close-packed directions. At about 890°C, the titanium undergoes an allotropic transformation to a body-centered cubic β phase which remains stable to the melting temperature (Figure 1).
All elements which are within the range 0.85-1.15 of the atomic radius of titanium alloy substitutionally and have a significant solubility in titanium. Elements with an atomic radius less than 0.59 that of Ti occupy interstitial sites and also have substantial solubility (e.g. H, N, O, C). The ease with which solutes dissolve in titanium makes it difficult to design precipitation-hardened alloys. Boron has a similar but larger radius than C, O, N and H; it is therefore possible to induce titanium boride precipitation. Copper precipitation is also possible in appropriate alloys.
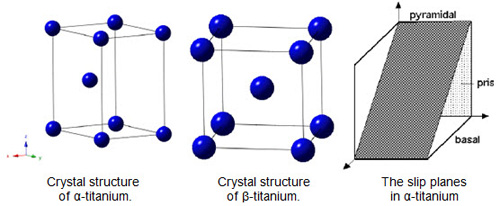
Figure 1: Crystal structures of Titanium
The alloying elements can be categorized according to their effect on the stabilities of the α and β phases. Thus, Al, O, N and Ga are all α-stabilizers. Mo, V, W and Ta are all β-stabilizers. Cu, Mn, Fe, Ni, Co and H are also β-stabilizers but form the eutectoid. The eutectoid reaction is frequently sluggish (because of substitutional atoms involved) and is suppressed.
Molybdenum and vanadium have the largest influence on β stability and are common alloying elements. Tungsten is rarely added due to its high density. Cu forms TiCu2 which makes the alloys age-hardening and heat treatable; such alloys are used as sheet materials. It is typically added in concentrations less than 2.5 wt% in commercial alloys. Zr, Sn and Si are neutral elements.
Interstitials
These do not fit properly and cause changes in the lattice parameters. Hydrogen is the most important interstitial. Body-centered cubic Ti has three octahedral interstices per atom whereas c.p.h. Ti has one per atom. The latter are therefore larger, so that the solubility of O, N, and C is much higher in the α phase.
Because of this characteristic, titanium is a candidate material for the first wall of magnetically confined fusion reactors. The hydrogen based plasma is not detrimental since at 500°C and 1Pa pressure, the Ti does not pick up enough hydrogen for embrittlement. An additional feature is that Ti resists swelling due to neutron damage.
A large enough concentration of hydrogen induces the precipitation of hydrides. TiH1.5-2.0 has a Cubic-F lattice and its precipitation causes embrittlement due to a volume expansion of about 18%. There are regions of hydrostatic tension at crack tips where it forms preferentially, leading to large increases in the crack growth rate, some 50-fold during fatigue.
One problem with this method of hydrogen storage is that hydride formation is accompanied by a considerable volume expansion, which in turn can embrittle the alloy. Amorphous alloys of titanium are better in this respect, since they do form hydrides and yet reversibly accommodate large quantities of hydrogen by an expansion of the nearest-neighbor distance. Titanium and zirconium are metallurgically similar. The latter also forms hydrides.
Titanium Aluminide
Titanium aluminides are attractive structural materials for the aerospace industry due to their low density, high specific strength and modulus retention and excellent creep resistance. They have been substituted for nickel alloys in the turbines of military aircraft. There are two major forms - α2 and gamma-based titanium aluminides although interest is fading in the α2 alloys for many applications.
The tensile ductility is about 4-6% at ambient temperature. The γ-aluminide tends to be more ductile. The density is about 4.5 g cm2 and the aluminum makes the aluminide more resistant to burning. The alloys have been studied extensively for aerospace and automotive turbochargers because of their high strength, low density and creep resistance.
Standards
About 50 grades of titanium and titanium alloys are designated and currently used, although only a couple of dozen are readily available commercially. The ASTM International recognizes 31 grades of titanium metal and alloys, of which Grades 1 through 4 are commercially pure (unalloyed).
The grades covered by ASTM and other alloys are also produced to meet Aerospace and Military specifications (SAE-AMS, MIL-T), ISO standards, British Standards Institution (BSI), Deutsche Institut fur Normalforschung (DIN), GOST for the FSU and the Japanese Institute of Standards. Although there is overlap between these standards they are not interchangeable. The Japanese Titanium Society in its efforts to achieve a more global approach to titanium has made representations for unified systems of specifications.
There are established standards for the industry. However, the major problem is not a lack of standards but the detailed, individual specifications by the aerospace companies which limit production runs. Each aerospace company has its own specification which includes processing routes. Consequently a process route which is allowed by Rolls-Royce may not be allowed by Boeing. This results in small batch production.
Over production can be stored but can only be used by a company which agrees the specification and the material is probably over-qualified for industrial use having gone through the very detailed quality assurance requirements of the aerospace industry. Similarly the high proportion of material which results as scrap must be carefully separated in order to provide the detailed pedigree which is required by the aerospace companies.
Access Precise Properties of Titanium Alloys Now!
Total Materia Horizon contains property information for thousands of titanium alloys: composition, mechanical and physical properties on various temperatures, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.