Bulk Metallic Glass: Part One
Abstract
Even as other materials researchers have revolutionized industries with composites and 3D printed polymers, metallurgists have been searching for a transformative advance in metals.
Amorphous metal, aka metallic glass, has the potential to revolutionize the use of metals, if only a viable process can be found that will overcome its daunting production challenges.
An amorphous metal (also known as metallic glass) is a solid metallic material, usually an alloy, with a disordered atomic-scale structure. Most metals are crystalline in their solid state, which means they have a highly ordered arrangement of atoms. Amorphous metals are non-crystalline, and have a glass-like structure. But unlike common glasses, such as window glass, which are typically electrical insulators, amorphous metals have good electrical conductivity. There are several ways in which amorphous metals can be produced, including extremely rapid cooling, physical vapor deposition, solid-state reaction, ion irradiation, and mechanical alloying.
In the past, small batches of amorphous metals have been produced through a variety of rapid cooling methods. For instance, amorphous metal ribbons have been produced by ejecting molten metal onto a spinning metal disk (melt spinning). The rapid cooling, on the order of millions of degrees a second, is too fast for crystals to form and the material is "locked" in a glassy state. More recently a number of alloys with critical cooling rates low enough to allow formation of amorphous structure in thick layers (over 1 millimeter) have been produced; these are known as bulk metallic glasses (BMG). More recently, batches of amorphous steel with three times the strength of conventional steel alloys have been produced.
As mentioned above conventional metallic materials have a crystalline structure consisting of single crystal grains of varying size arranged in a microstructure. Such structures are produced by the nucleation and growth of crystalline phases from the molten alloy during solidification. By contrast, certain oxide mixtures (e.g. silicate glasses), have such sluggish crystal nucleation and growth kinetics, that the liquid can be readily undercooled far below the melting point of crystals (e.g. a quartz crystal). At deep undercooling, these oxide melts undergo a "glass transition" and freeze as vitreous solids.
Bulk metallic glasses have unusual properties. They are typically much stronger than their crystalline metal counterparts (by factors of 2 or 3), are quite tough (much more so than ceramics), and have very high strain limits for Hookean elasticity (see Figure 1). A new class of engineering materials, BMG's offer an opportunity to revolutionize the field of structural materials with combinations of strength, ductility, toughness, and processability outside the envelope achievable using conventional technology.
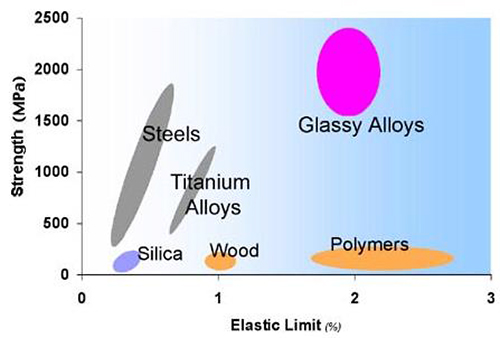
Figure 1: Typical strengths and elastic limits for various materials. Metallic glasses are unique.
Development of casting and processing methods for producing near net shape components of metallic glass and its composites is under way. Composites include extrinsic reinforcement, such as ductile fibers and particles, and in situ growth of beta-phase titanium dendrites in Vitreloy (Zr based) bulk metallic glass. As implied by Figure 2 below, toughening BMG requires adding impediments to shear band propagation which have a size and distribution comparable to the shear-band spacing. Figure 3 shows a die-cast BMG component used to fabricate a watch.
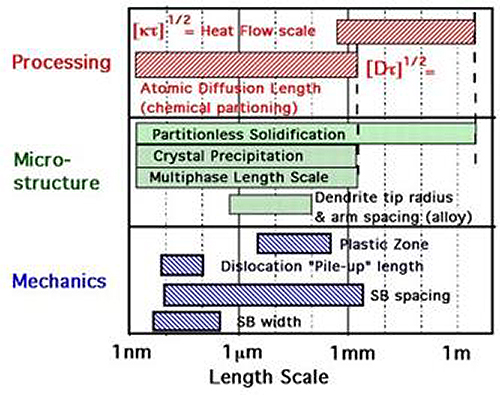
Figure 2: Some length scales for physical phenomena in the structure, processing and properties of materials.

Figure 3: A net-shaped cast of a watch case directly out of the mold with no finishing or polishing.
Bulk metallic glasses (BMG) have found many wide and diverse applications in industry, from more durable mobile phones and other electronic equipment to, golf club cases and more recently increasingly durable biomedical applications such as the implantation into bones of screws, pins, or plates to fix fractures.
Find Instantly Precise Material Properties!
Total Materia Horizon contains mechanical and physical properties for hundreds of thousands of materials, for different temperatures, conditions and heat treatments, and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.