Corrosion of Zirconium Alloys: Part Two
Abstract
Zirconium is a common choice for nuclear applications due to its low thermal neutron capture cross section which is about 30 times less than that of stainless steel.
A specific key useful benefit of zirconium alloys are their specific resistance to acids including hydrochloric, sulfuric and phosphoric acids which means applications which require direct contact with these substances is particularly suited.
The main properties of Zr and the Zr alloys are given in Table 1. It should be noted that one of the main reasons for selecting Zr as a nuclear material is its low thermal neutron capture cross section which is about 30 times less than that of stainless steel giving a better neutron efficiency in water reactors. The main characteristics of Zr metallurgy come from its high reactivity with oxygen, from the different type of chemical interactions with the alloying elements (complete solubility or intermetallic compound formation) and from its strongly anisotropic hexagonal crystal structure, the latter leading to the development of a textured material after thermo-mechanical processing.
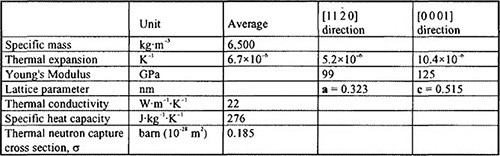
Table 1: Physical Properties of the Zr Alloys
Zirconium has outstanding resistance to hydrochloric acid, sulfuric acid, organic acids, and alkaline media such as sodium hydroxide. Its resistance to nitric acid is equaled only by the noble metals such as tantalum. The most common application areas for cast zirconium equipment are in hydrochloric acid, sulfuric acid, and hot organic acids. Zirconium shows excellent corrosion resistance to all concentrations of hydrochloric acid even at temperatures exceeding the normal boiling point. However, zirconium is not resistant to hydrochloric acid containing oxidizing species such as cupric chloride, ferric chloride, or wet chlorine. Zr 702C is resistant to sulfuric acid concentrations up to 70 percent and Zr 705C is resistant to concentrations up to 55 percent to the normal boiling point of sulfuric acid. Poor resistance is obtained with higher concentrations, even at room temperature.
Zirconium is superior to stainless steels, nickel alloys, and titanium in organic acids. This alloy is considered for these applications at high temperatures where its marked superiority results in a distinct economic advantage. Zirconium has poor resistance to concentrated sulfuric acid, hydrofluoric acid, concentrated phosphoric acid, ferric chloride, cupric chloride, wet chlorine, and other oxidizing chloride environments.
One of the earliest applications for zirconium was in the handling of hydrochloric acid. Zirconium is still widely used in this area. The corrosion rate of zirconium is less than 5mpy at all concentrations and temperatures well in excess of the boiling point. Even in 37% HCI, zirconium does not corrode appreciably until temperatures of 130°C are reached (see Figure 1). Zirconium’s corrosion-resistant behavior in pure hydrochloric acid is superior to any other engineering metal.
Aeration does not affect zirconium’s behavior in hydrochloric acid. However, oxidizing impurities such as cupric or ferric chlorides in relatively small amounts will decrease the corrosion resistance of zirconium in hydrochloric acid. These oxidizing ions should be avoided or electrochemical protection methods should be used to counteract the effect of oxidizing impurities.
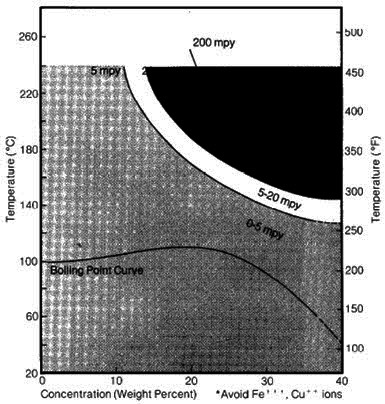
Figure 1: Corrosion of Zircadyne 702 in hydrochloric acid solutions
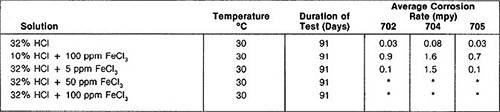
Table 2: Corrosion of Zircadyne 702 in hydrochloric acid solutions
Applications of zirconium in hydrochloric acid
Current applications of zirconium equipment in hydrochloric acid include pumps, valves, piping, condensers, and evaporators. Zirconium heat exchangers, pumps, and agitators have been used for over 20 years in an azo dye coupling reaction. Besides being very corrosion resistant in this environment, zirconium does not plate out undesirable salts which would change the color and stability of the dyes. Heat exchangers made of zirconium are used by chemical companies in the production of polymers. Other hydrochloric applications where zirconium has been used are phthalic-hydrochloric acid and wet hydrogen chloridechlorine atmospheres. A very important application for zirconium is in processes that cycle between hydrochloric or sulfuric acid and alkaline solutions.
Applications of zirconium in sulfuric acid
Zirconium is used extensively in 40-70 weight percent sulfuric acid at elevated temperatures. Zirconium has been used to replace impervious graphite heat exchangers and lead heating coils in an environment containing sulfuric acid, zinc and sodium sulfates, hydrogen sulfide, and carbon disulfide. Zirconium has been used in these applications for over ten years without corrosion problems. Some other examples of sulfuric acid applications include ester manufacturing, steel pickling, alcohol stripping towers and hydrogen peroxide manufacturing.
Phosphoric acid
Zirconium is corrosion resistant in phosphoric acid at concentrations up to 55% and temperatures exceeding the boiling point. Above 55% H3PO4, concentrations the corrosion rate rises with increasing concentration and temperature (see Figure 10). The corrosion rate of zirconium is still less than 5mpy at 60°C and concentrations up to 85%. Fluoride ion impurities in phosphoric acid can cause attack of zirconium.
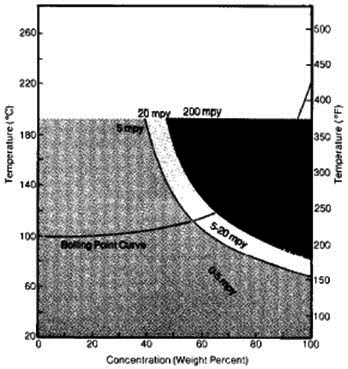
Figure 2: Corrosion of zirconium in phosphoric acid solutions
Read more
Access Precise Corrosion Properties Now!
Total Materia Horizon contains corrosion behaviour and property information for hundreds of thousands of materials, accross more than 2,000 media.

Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.