Boron in Steel: Part One
Abstract
At the atmospheric pressure and temperature of 0°C boron is a solid material. Boron optimum quantity which has to be added in the steel to achieve maximum hardenability, based on experience is about 0.0003 to 0.0030% B.
Boron is supplied to steelmakers as ferroboron or as one of several proprietary alloys. Choice of addition depends, as always, on steelmaking practice, product mix and volume, individual operators` experience and preference, and price.
The Basic Properties of Boron
The name "boron" originates from the Armenian-Persian linguistic area: buraq or burah for borax, one of the most widely-known boron compounds, namely sodium sodium tetraboratedecahydrate. As long ago as the Middle Ages borax was exported under the name "Tincal" from the salt lakes of Central Asia to Europe, where it was used as an aid in soldering and melting.
The proportion of boron in the earth’s outer crust is estimated to be to 10ppm and in nature boron does not occur in the elementary state, but always combined with oxygen. The technically most important boron-containing minerals are:
- Borax (Tincal): Na2O x 2B2O3 x 10H2O;
- Tincalconite: Na2O x 2B2O3 x 5H2O;
- Kernite (Rasorite): Na2O x 2B2O3 x 4H2O;
- Boracite: 5MgO x MgCl2 x 7B2O3;
- Colemanite: 2CaO x 3B2O3 x 5H2O;
- Sassolin: B2O3 x 3H2O.
The greatest deposits of boron are located in Kazakhstan, California, Argentina and Turkey. Boron was first successfully produced in 1808 by H. Davy, as amorphous. Boron is the only nonmetal of the third main group of the periodic table. At the atmospheric pressure and temperature of 0°C boron is a solid material. It has six isotopes, some of which are radioactive with very short time of semi decay, below 1 second. Some of the most important properties of boron are listed in the tables 1, 2 and 3.
| Modification | Amorphous | Crystaline |
| Color | Brown | Black-grey |
| Phase | α β | γ |
| Temperature of occurrence, °C | 800-1100 ≥1300 | 1100-1300 |
| Lattice type | rhombohedral | tetragonal |
| a=1,789 nm | ||
| b=0,895 nm | ||
| c=1,015 nm |
Table 1: Boron: structural data
| Amorphous | Crystaline | |
| Density, g/cm | 1.73 | α mod. 2.46 β mod. 2.35 γ mod. 2.37 |
| Hardness | 9.3 | |
| Tensile strength, MPa | amorphous 1.6-2.4 fibres 2.6-3.1 |
|
| Compressive strength, MPa | 0.5 | |
| Elasticity modul, MPa | 440 |
Table 2: Boron: mechanical properties
| Flame point, °C | 70 |
| Melting point, °C | 2300 |
| Boiling point, °C | 2550 |
| Coefficient of thermal expansion | 20-750 1.1- 8.3 nm/m.k |
| Latent heat of vaporization | 34900 kg/kg |
| Latent heat of fusion | 22000 kJ/kg |
| Latent heat of combustion | 5.4 kJ/kg |
| Diffusion coefficient in γ-Fe | D= 0.002 e-21000RT for t=1000 C : D=0.002 |
Table 3: Boron: thermodynamic data
According to P.E. Brushby et al. boron diffusion velocity is the same as carbon diffusion velocity (diffusion coefficient D=0,002e-21000/RT). With carbon content of up to 0.43%, the solubility of boron in the austenite lattice is independent of the carbon content. Carbon diffusion is not affected up to boron contents of 0,009%.
Boron Solubility in Pure Iron
There are different opinions about the positions of boron atoms in the iron crystal lattice. Based on comparison of the diffusion coefficients of carbon and boron, Jandeska and I.J.Morral have concluded that the boron atoms are interstitially dissolved in the γ-Fe lattice. C.C. McBride, J.W. Spretnak, R.Speiser have reached the same results based on comparison of the theoretical consideration of the boron atoms radius and interatomic distances in the γ-Fe lattice. However, these geometric considerations ignore the mechanisms of physical and chemical bonding.
R.M.Goldhoff and J.W.Spretnak found by X-ray deflection measurements that the lattice parameter in gamma iron is reduced in the presence of boron. They took this as a proof of a substitutional dissolution of the boron atoms in the austenite lattice. Due to the atomic radii of boron and iron, they considered the position of the boron atoms at the lattice locations to be more favorable than at intermediate lattice locations.
They also discovered that the difference between the lattice parameters of pure iron and boron-containing iron decreases with increasing temperature. From this they concluded that with increasing temperature more boron atoms migrate from the lattice to the grain boundaries, where boron separations have actually been found. However, these authors did not exclude the possibility that a smaller number of boron atoms may also occupy intermediate lattice locations.
There is several different works on the Fe-B binary system. Figure 1 shows a diagram prepared by O.Kubaschevsky. It shows two eutectics, one at 17 atomic percent of boron, the other at 63.5% of boron.
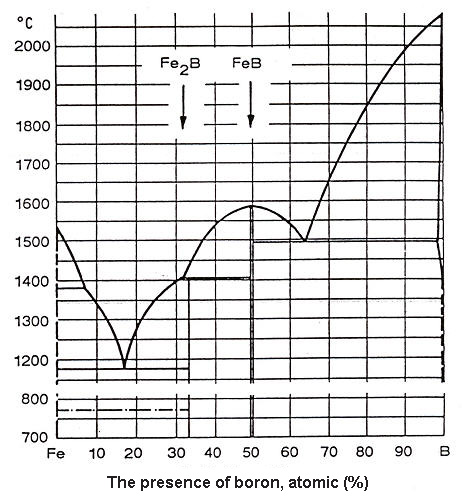
Figure 1: Kubaschewsky Fe-B phase diagram
Within the range between these two eutectics, the liquidus temperature varies between 1149°C, the eutectic temperature at 17 atomic percent of boron, and 1590°C for the alloy with 50 atomic percent of boron. Accordingly, the melting temperature of iron can be lowered by more than 150°C to the maximum of 350°C (at 17 atomic percent of boron) by the adding of 5 to 30 atomic percent of bor. By increasing proportion of boron in the ferroboron from the second eutectic, the liquidus temperature rises steadily and almost linearly up to the melting temperature of pure boron. Accordingly, in the above diagram, iron boride (FeB) has 16.23 weight percent of boron, a rhombic lattice and extreme hardness of 2300 HV0.2
Iron-II-boride (Fe2B) has 8.83 weight percent of boron, a tetragonal lattice and hardness of 1800 to 2000 HV0.2 (preferable as long as FeB is being avoided). Many authors have made more precise investigations of the iron/boron binary system in the iron rich corner. According to Houndremon's binary phase diagram Fe-B (Figure 2), in the low concentration region of boron the maximum solubility of boron is 0,021% at 1149°C. In the meantime, with the temperature decrease its solubility decreases too, as far as down to 0.0021% at 906°C.
At the same time this temperature is perytectoide for perytectoide reaction at 0.0082%B. Boron solubility in γ-Fe suddenly decreases and at 710°C only about 0.0004% of boron substitionally dissolved.
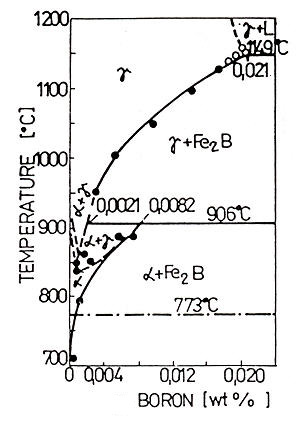
Figure 2: Equilibrium phase Fe-B diagram for low boron concentrations
J.W.Spretnak investigated whether it is possible for boron to form a film surrounding austenitic grain in the steel and concluded on the basis of the geometrical considerations that it is impossible. Instead, they discovered a point Fe2B precipitation at the grain boundaries. In the steel, boron can be dispersed in matrix in the form of Fe2B, boride with size of 20 to 30x10-8 cm and free, which segregates predominantly surrounding primary austenite grain boundaries. This small amount of the soluble boron arranged along grain boundaries, evidently retards γ-α transformations by diffusion, namely it prevents feritic reaction thus enhancing hardenability of the steel.
Boron optimum quantity which has to be added in the steel to achieve maximum hardenability, based on experience is about 0.0003 to 0.0030% B. Boron addition beyond the mentioned values deteriorates hardenability because the excess of boron atoms precipitates as the surface centered cube Fe23(CB)6 borocarbide which can be a ferrite nucleation preferential place.
Available Forms
Boron is supplied to steelmakers as ferroboron or as one of several proprietary alloys. Choice of addition depends, as always, on steelmaking practice, product mix and volume, individual operators' experience and preference, and price. A steelmaker should choose that addition agent giving the highest and most reliable recovery consistent with his overall melt shop economics.
Ferroboron is the lowest cost addition agent. Boron content is relatively high: standard grades are sold with incremental boron levels between 12 and 24% B. Major impurities are carbon (0.10-1.5%), silicon (0.30-4.0%) and aluminum (0.5-8.0%). A typical analysis will include 18.0% B, 0.50% C, 0.50% Si, 0.2% Al, 0.03% P, and 0.01% S. All except boron are maximum values. Product is supplied in lump form, 2 in. or 1 in. x down, packaged in 250 kg or 500 lb steel drums, or supersacks (bulk bags) of up to 3000 lb (1360 kg) capacity. Many customers apply a minimum size limit, such as 5 mm (0.2 in.), in order to minimize the amount of fine material, which can give poor recoveries in less well-controlled melting practices. Ferroboron is also available as cored wire.
Because ferroboron does not contain appreciable concentrations of protective elements, it requires greater care than the proprietary alloys in order to give adequate and consistent results. It is normally added after other oxygen/nitrogen scavengers, such as ferrotitanium.
The proprietary boron addition agents are more expensive than ferroboron on an initial cost basis but are often preferred for their greater efficiency, ease of application and more consistent results. All will contain varying proportions of oxygen and/or nitrogen scavengers such as titanium, aluminum, silicon and zirconium. These elements generally have an even greater affinity for oxygen and nitrogen than boron.
The most common proprietary addition agent typically contains 2.0% B, 15% Al, 30% Ti, 10% Si, bal. Fe. This product's high scavenger/boron ratio ensures its effectiveness for all boron steels, provided they have been adequately deoxidized first.
A variety of other composition proprietary boron addition alloys are available, with boron contents varying between 0.5% and 4%. Generally, the higher the ratio of boron to scavenger elements, the greater the care required to ensure adequate recovery of the boron in the steel.
Proprietary boron addition agents are sold in lump form 1-1/4 in. and 2 in. x down, packaged in bags, cans or large drums.
Read more
Find Instantly Precise Compositions of Materials!
Total Materia Horizon contains chemical compositions of hundreds of thousands materials and substances, as well as their mechanical and physical properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.