Copper-Tin Alloys: The Bronzes
Abstract
This article examines copper-tin alloys, commonly known as bronzes, which have significant industrial applications within specific tin content ranges. The constitutional diagram of these alloys reveals a complex relationship between composition and microstructure. As tin is added to copper, various solid solutions form (designated as α, β, γ, etc.), each with distinct properties. The article explores the microstructural characteristics and properties of three important bronze compositions: 95:5, 90:10 (gun-metal), and 85:15 (bearing and bell metal). Understanding these microstructural changes during solidification and cooling is essential for controlling the mechanical properties of bronze alloys in industrial applications.
Introduction to Bronze Metallurgy
The important alloys of copper and tin from an industrial perspective are the bronzes, which exist within certain limits of tin content. Similar to brasses, adding tin to copper results in the formation of a series of solid solutions. While the complete constitutional diagram of copper-tin alloys is quite complex, Figure 1 illustrates the portion relevant to industrial applications.
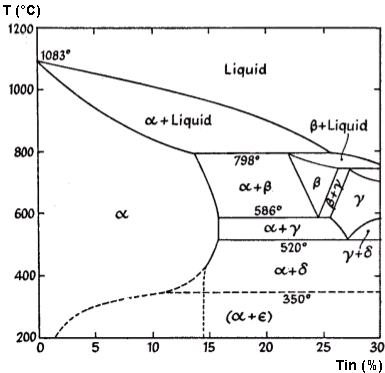
Figure 1: Constitutional Diagram of the Copper-Tin Alloys
When tin is added to copper, it creates a series of solid solutions that are conventionally designated in order of decreasing copper content as α, β, γ, and so forth. The constitutional diagram can be summarized as follows:
Table 1. Phase composition of copper-tin alloys at different temperatures
| Percentage composition | Constituent just below the freezing point | Constituent after slow cooling to 400°C | |
| Copper | Tin | ||
| 100 to 87 | 0 to 13 | á | á |
| 87 to 86 | 13 to 14 | á + â | á |
| 86 to 78 | 14 to 22 | á + â | á + ä |
| 78 to 74 | 22 to 26 | â–>(á + â) | á + ä |
Further transformations during cooling from 400°C to room temperature occur so slowly that they rarely happen under normal manufacturing conditions.
Microstructural Constituents of Bronze
The α solution represents the softest constituent in the copper-tin system. While it can be cold-rolled or stamped, it work-hardens more rapidly than α-brass during cold working.
In slowly cooled alloys, the β and γ constituents do not persist to room temperature. This is due to phase transformations occurring at 586°C and 520°C, where β decomposes into α + γ, and γ transforms into α + δ.
The δ constituent, with a crystal structure similar to α-brass, exists within a narrow composition range approximating Cu31Sn8. Like most intermetallic compounds, it exhibits extreme hardness and brittleness. The δ → (α + ε) transformation at 350°C rarely occurs in commercial practice.
Alloys with higher tin content may contain the ε constituent (corresponding to Cu3Sn) and the ζ solid solution (approximately CuSn in composition).
Commercial Bronze Compositions
95:5 Copper-Tin Alloy (Coinage Bronze)
When cooling from the liquid state, the first solid solution that forms contains only about 2% tin. This creates a pronounced cored structure in the cast metal due to the wide temperature gap between liquidus and solidus. This coring can be eliminated through slower cooling or by annealing.
Oxygen absorption during manufacturing results in SnO2 formation in the alloy, which tends to increase brittleness. Therefore, deoxidizers such as zinc are frequently added. Zinc addition, common in coinage bronze, does not alter the microstructural appearance of the homogeneous α constituent but serves as an effective deoxidizer in the liquid state while slightly hardening the solid solution.
Bronze coins typically display marked deformation of crystals in their structure. Upon annealing, recrystallization occurs followed by crystal growth, with twinning being a characteristic feature of cold-worked and annealed alloys.
90:10 Copper-Tin Alloy (Gun-Metal)
This composition represents typical gun-metal, though most commercial varieties contain deoxidizing elements, often zinc (e.g., Admiralty gun-metal: 88% copper, 10% tin, 2% zinc). The microstructure of cast gun-metal depends significantly on cooling rates during and after solidification.
Due to the alloy's wide solidification range and slow tin diffusion, the apparent solubility limit of the α solution falls below what the equilibrium diagram indicates. The cast structure invariably shows distinct dendrites, and if coring is pronounced, some β solution may form at 798°C. This interdendritic β transforms during cooling into the hard δ constituent. Conversely, slow cooling or prolonged annealing can produce a homogeneous α microstructure. Consequently, chill-cast gun-metal differs substantially in both structure and properties from annealed gun-metal.
85:15 Copper-Tin Alloy (Bearing and Bell Metal)
This composition is typical for various bronze bearing metals, most containing small zinc additions as deoxidizers. It also approximates the composition of traditional bell metal.
Immediately after solidification, this alloy consists of α and β constituents. Rapid cooling preserves these phases, while slow cooling causes the β (or γ) constituent to completely transform below 520°C into a complex α + δ microstructure. In slowly cooled alloys, the α + β structure is replaced by α + (α + δ) complex. This microstructural difference explains why sand castings of this alloy are considerably harder than chill castings, and provides the metallurgical basis for heat treatment methods applied to both bells and bearing components.
Access Precise Properties of Copper Alloys Now!
Total Materia Horizon contains property information for 30,000+ copper alloys: composition, mechanical, physical and electrical properties, nonlinear properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.