Clean Steel: Part Three
Abstract
This article, the third in a series on clean steel, examines how non-metallic oxide inclusions significantly impact steel quality. These inclusions degrade mechanical properties, reduce ductility, and increase failure risks in finished products. The article explores inclusion formation mechanisms, focusing on calcium treatment to modify alumina inclusions into liquid calcium aluminates. This modification improves continuous casting processes, reduces nozzle blockage, and enhances various mechanical properties. The analysis covers the thermodynamics of calcium reactions, optimal treatment conditions, and limitations in specific applications like automotive sheet steel where alternative cleanliness methods are required.
Introduction to Non-Metallic Inclusions in Steel
The presence of non-metallic oxide inclusions represents a major challenge in achieving desired cleanliness levels in commercial steel grades. These inclusions generally degrade the mechanical properties of steel, reducing the ductility of cast metal and increasing the risk of mechanical and/or corrosion failure in final products.
In recent years, increasing demand for high-quality steel products has driven continuous improvement in steelmaking practices. Special attention has focused on controlling non-metallic inclusions due to their harmful effects on subsequent processing stages and significant influence on final product properties. By controlling the amount, size, and chemical composition of inclusions, manufacturers can obtain a final product of superior quality. Therefore, understanding inclusion formation mechanisms and identifying their constituent phases are critically important for producing clean steels.
Sources and Types of Oxide Inclusions
Oxide inclusions originate from two primary sources:
- Endogenous (micro) inclusions: Residual products resulting from intentionally added alloying elements used to deoxidize the molten steel after oxygen treatment.
- Exogenous (macro) inclusions: Products resulting from reactions between the melt and atmosphere, slag, or refractory materials.
Among various types of non-metallic inclusions, oxide and sulfide inclusions are considered particularly harmful for common steel grades.
Alumina Inclusions and Calcium Treatment
Alumina inclusions occur as deoxidation products in aluminum-based deoxidation of steel. Pure alumina has a melting point above 2000°C, meaning these inclusions remain solid in liquid steel. The addition of calcium to steel containing such inclusions transforms their composition from pure alumina to CaO-containing calcium aluminates.
As shown in Figure 1, the melting point of calcium aluminates decreases as the CaO content increases, with liquid oxide phases appearing at approximately 22% CaO content (when the CaO·2Al₂O₃ compound is first exceeded at 1600°C). The liquid phase content continues to increase with rising CaO content, reaching 100% at 35% CaO. The minimum melting temperature for liquid calcium aluminates is around 1400°C, meaning such inclusions may remain in liquid form until or even after steel solidification.
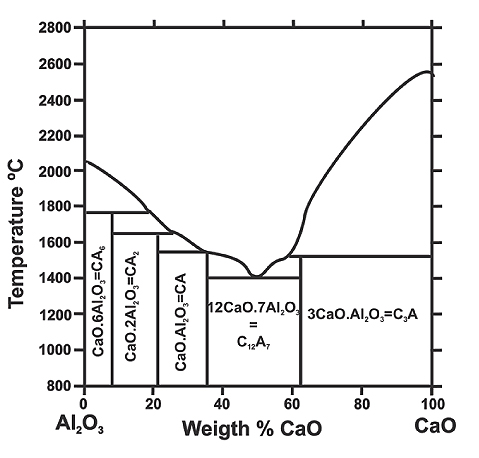
Figure 1: Binary system CaO-Al₂O₃
Calcium Treatment Methods and Mechanisms
Most steel grades undergo calcium treatment using either a Ca-Si alloy or a Ca-Fe(Ni) mixture, depending on the silicon specification. This treatment occurs after trim additions and argon rinsing.
In most melt shops, the calcium treatment utilizes cored wire containing Ca-Si or Ca-Fe(Ni) injection systems. Calcium has melting and boiling points of 839°C and 1500°C respectively. During treatment, alumina and silica inclusions convert to molten calcium aluminates and silicates, which become globular due to surface tension effects. This transformation in inclusion composition and shape is known as inclusion morphology control.
The calcium aluminate inclusions retained in liquid steel suppress the formation of MnS stringers during steel solidification. This change in composition and precipitation mode of sulfide inclusions is known as sulfide morphology or sulfide shape control.
Metallurgical Advantages of Calcium Treatment
Several metallurgical advantages result from modifying the composition and morphology of oxide and sulfide inclusions through calcium treatment:
- Improved steel castability in continuous casting, minimizing nozzle blockage
- Reduced inclusion-related surface defects in billet, bloom, and slab castings
- Enhanced steel machinability at high cutting speeds with prolonged carbide tool life
- Decreased susceptibility to reheat cracking in weld heat-affected zones
- Prevention of lamellar tearing in large restrained welded structures
- Reduced susceptibility of high-strength low-alloy (HSLA) linepipe steels to hydrogen-induced cracking in sour environments (Ca content can be controlled within 15-20 ppm)
- Increased tensile ductility and impact energy in transverse and through-thickness directions in steels with tensile strengths below 1400 MPa
Thermodynamics of Calcium Reactions
When calcium is injected deep into the melt, the following series of reactions occur to varying extents in Al-killed steels containing alumina inclusions:
Ca + O = CaO (1)
Ca + S = CaS (2)
Ca + (x+1/3)Al₂O₃ = CaO·x Al₂O₃ + 2/3[Al] (3)
Depending on steel composition, calcium addition methods, and other process variables, there will be variations in the conversion of alumina inclusions to aluminate inclusions. Smaller inclusions convert to molten calcium aluminates more readily than larger ones.
From a thermodynamic perspective, if sulfur or oxygen is dissolved in steel at moderate levels, or if Al₂O₃ inclusions are present, calcium will react with oxygen or sulfur until the reactant contents become very low (< 2ppm). A critical question is whether calcium added to steel will react with sulfur via reaction (2) to form CaS or modify Al₂O₃ to liquid calcium aluminates via reaction (3).
Calcium sulfide formation occurs when calcium and sulfur contents are sufficiently high. Since calcium has higher affinity for oxygen than for sulfur, initial calcium addition results in conversion of alumina into calcium aluminates until calcium sulfide formation begins as additional calcium is introduced.
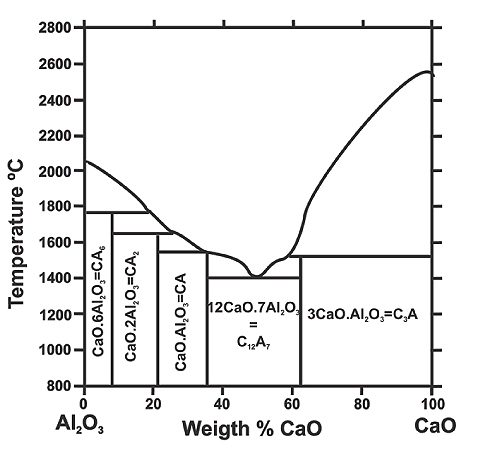
Figure 2: Change of inclusions composition during calcium additions
As observed in Figure 2, alumina conversion to calcium aluminates continues until all inclusions in the steel are present only in liquid form.
Preventing Nozzle Clogging in Continuous Casting
To prevent nozzle clogging in continuous casting caused by solid inclusions, calcium is added to steel to modify inclusions and desulfurize the steel. Calcium converts solid alumina inclusions into lower melting point calcium aluminates, helping prevent casting nozzle clogging. However, calcium also reacts with oxygen and sulfur, modifying sulfide inclusions. If the steel's sulfur content is high, calcium will react with sulfur to form solid CaS, which could potentially clog continuous casting nozzles.
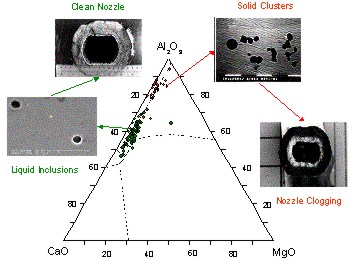
Figure 3: Influence of calcium treatment on the type of inclusions formed and its relationship with nozzle clogging
Figure 3 illustrates how calcium treatment influences the types of inclusions formed and their relationship with nozzle clogging.
Limitations of Calcium Treatment
Calcium treatment cannot be universally applied to all steel types. For steels requiring high formability, such as automotive sheet, calcium treatment is unsuitable because it produces hard calcium aluminate inclusions. For these steel types, improving molten steel purity is the preferred approach to optimize castability. Through controlling carry-over slag from melting furnaces, deformation treatment of ladle slag, metallurgy in tundish, protective casting, and other measures, steel purity can be maintained while reducing total oxygen content in the molten steel.
Read more
Find Instantly Precise Compositions of Materials!
Total Materia Horizon contains chemical compositions of hundreds of thousands materials and substances, as well as their mechanical and physical properties and much more.
Get a FREE test account at Total Materia Horizon and join a community of over 500,000 users from more than 120 countries.